Translate this page into:
The outcome of proliferative lupus nephritis with pulse cyclophosphamide therapy
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Proliferative lupus nephritis deserves aggressive therapy and cyclophosphamide plays a pivotal role. Thirty nine patients with proliferative lupus nephritis (Class III-7 patients and Class IV- 32 patients) with a median follow up of 38 months were considered for this observational study. All the patients received induction therapy with intravenous methylprednisolone. Cyclophosphamide was given intravenously initially in monthly pulses for six months and later quarterly pulses until remission was achieved or until the target dose (200 mg/kg) was reached. The treatment with intravenous methylprednisolone was repeated in the event of a nephritic flare. Later the corticosteroid was reduced to a minimum effective dose and cyclophosphamide was changed to either azathioprine or mycophenolate mofetil. At the time of the last follow up, 82.05% of the patients were in remission (complete remission 51.28% and partial remission 30.77%). The median interval to achieve remission in responders was 15 months. Early diagnosis (P=0.04), a higher creatinine clearance at presentation (P=0.02), and concurrent use of an ACEI or an ARB (P=007) significantly favored attaining remission. Five patients experienced a doubling of serum creatinine and one of them became dialysis dependent. Risk of doubling of serum creatinine correlated with a low Ccr (P=0.03) at presentation, occurrence of renal flares (P=0.034) and failure to achieve remission (P=0.0001). The parameters like serum creatinine, serum C3, serum C4, activity and chronicity indices on renal biopsy, hypertension were not statistically significant. Therapy with cyclophosphamide, if initiated early, helps in inducing remission and hence can retard the progression to CKD.
Keywords
Chronic kidney disease
cyclophosphamide
lupus nephritis
remission
Introduction
Systemic lupus erythematosus (SLE) is a chronic, life threatening disease that predominantly affects women of childbearing age. Nephritis complicates SLE in approximately 25–50% of patients and is associated with increased mortality.[1]
There are few diseases for which the cause, natural history, and response to treatment have been as complex or difficult to define as those of SLE. In large part, this is because SLE represents a clinical syndrome rather than a single disease entity.
The quest for an ideal therapy that can provide a long-term remission with minimal side effects and prevent progression to chronic kidney disease (CKD) has remained elusive.[2] Cyclophosphamide has remained the mainstay in the treatment of lupus nephritis[3] and it is against this drug the other therapies are compared. Pulse intravenous cyclophosphamide has been preferred in most centers to continuous oral cyclophosphamide, because it appeared to be associated with less toxicity and is also preferred to mycophenolate mofetil (MMF) for financial constraints at least in this part of the world. Studies involving subjects from the Indian subcontinent have been far and few.[4] In this paper, we report the response of biopsy-proven patients with proliferative lupus nephritis to pulse cyclophosphamide.
Materials and Methods
This was an observational study undertaken in the Department of Nephrology, Nizam's Institute of Medical Sciences, Hyderabad between January 1998 and December 2009. Patients with biopsy proven (LM and IF) class III or Class IV lupus nephritis (ISN/RPS classification) who have received pulse cyclophosphamide and had a follow-up of at least three years were analyzed. The only exclusion criterion was the presence of significant chronicity (CI>2) changes on biopsy. Patients with features of membranous nephropathy were excluded.
All the patients had received methylprednisolone 1 g a day intravenously for three days before receiving cyclophosphamide intravenously as once a month pulse for the first six months. This was followed by quarterly pulses of cyclophosphamide until remission or target dose (200 mg/kg) was achieved. The dose of cyclophosphamide was 0.75 -1 g/m2 BSA in patients with normal renal function. The dose was halved to 0.375 – 0.5 g/m2 in patients who had an eGFR of < 30 ml/min. Mesna was administered to all patients receiving cyclophosphamide. Prednisolone (0.5 mg/kg/day) was given orally for six months. Then the dose was tapered gradually to 0.2 mg/kg/day over the next six months. Later this dose was administered on alternate days in those who achieved remission. Patients were continued on either MMF (1 g to 3 g a day as tolerated) or azathioprine (AZA) (1.5 to 2 mg/kg/day) after the completion of pulse cyclophosphamide therapy. Patients who suffered a nephritic flare were pulsed with methylprednisolone (1 g or 15 mg/kg in case of children) intravenously once a day for three days. There was no specific protocol regarding the use of ACEI or ARBs.
Doubling of serum creatinine was considered as the primary end point. The secondary end point was either death or the need for chronic dialysis.
The other definitions used were those proposed by the Renal Disease Subcommittee of the American College of Rheumatology.[5]
Nephritic flare was defined as a sudden increase in plasma creatinine of at least 30% over the last value associated with nephritic urinary sediment and increased proteinuria.
Proteinuric flare was identified when there was an increase in proteinuria without modification of plasma creatinine. Proteinuria had to increase by at least 2 g per day if the basal proteinuria was less than 3.5 g per day, or doubled, if the patient already had nephrotic proteinuria.
Complete renal remission was serum creatinine ≤1.2 mg/dl, and 25% increase of baseline creatinine clearance if abnormal, or stable value if normal at baseline, proteinuria <0.2 g/24 h, and inactive sediment defined as ≤5 red blood cells/high power field (hpf), ≤5 white blood cells/hpf and no cellular casts.
Partial renal remission was proteinuria from 0.21 to 2 g/day and serum creatinine ≤1.2 mg/dl, and 25% increase of baseline creatinine clearance if abnormal, or stable value if normal at baseline.
The glomerular filtration rate was calculated according to Cockcroft and Gault equation.
The statistical analysis was carried out with Sigma GraphPad Software, USA version-4. The data is presented as mean, standard deviation and median as indicated. For statistical significance, the probability value of less than 0.05 was considered. Survival curves were drawn using the Kaplan-Meier estimate and compared using the log-rank test. Contingency tables were analyzed using the Fischer's exact test.
Results
Thirty nine patients with proliferative lupus nephritis who had a follow up of at least three years (range: 36 to 124 months) were analyzed. The characteristics of patients at presentation are given in Table 1. The mean age at presentation was 27.35±9.75 (range 11 - 44)years. Females comprised the majority (F:M= 35:4). All the 39 patients were subjected to renal biopsy prior to the start of the therapy. The biopsy slides were reviewed and lupus nephritis was reclassified according to the ISN/RPN classification for the purpose of this analysis. Seven patients had class III lupus nephritis and the remaining 32 patients had class IV lupus nephritis. Patients considered having significant chronicity changes (CI>2) before the initiation of therapy were excluded from analysis. Anti dsDNA antibodies were detected in 30 patients (76.92%). The median interval between the diagnosis of SLE and lupus nephritis was 12 months and in eight patients the diagnosis was simultaneous. Renal failure (as defined under outcome measures and definitions) was present in nine patients (23.07%) at the time of presentation and one of these patients also had nephrotic proteinuria. Nine patients presented with nephrotic proteinuria alone (median 4.5 g/24h) and the rest presented with sub nephrotic proteinuria. Microscopic hematuria was present in 21 patients (53.85%).

All the patients had atl east three years of follow-up. The median follow up was 38 (range 36- 126) months. At the time of last follow up, 20 patients (51.28%) were in complete and 12 (30.77%) in partial remission. Median interval to achieve either complete or partial remission was 15 months. Two patients (5.13%) had neither achieved complete or partial remission though they had a decrease in proteinuria. One of these two patients had a decline from 10 g to 5.3 g and the other patient from 1.9 g to 1.4 g. The remaining five patients (12.82%) had progressed to the stage of chronic renal insufficiency (as defined under outcome measures and definitions) [Figure 1]. There were no deaths during the study period.

- Survival without doubling of serum creatinine
Among the various factors analyzed, the interval between the diagnosis of SLE and lupus nephritis, the Ccr and the use of ACEI or ARB were significant in predicting remission, either complete or partial. An early diagnosis of lupus nephritis and hence an early initiation of therapy helped in achieving remission. Similarly, the presence of a higher Ccr at the start of therapy and the concurrent use of either an ACEI or an ARB were also helpful in achieving remission [Table 2].
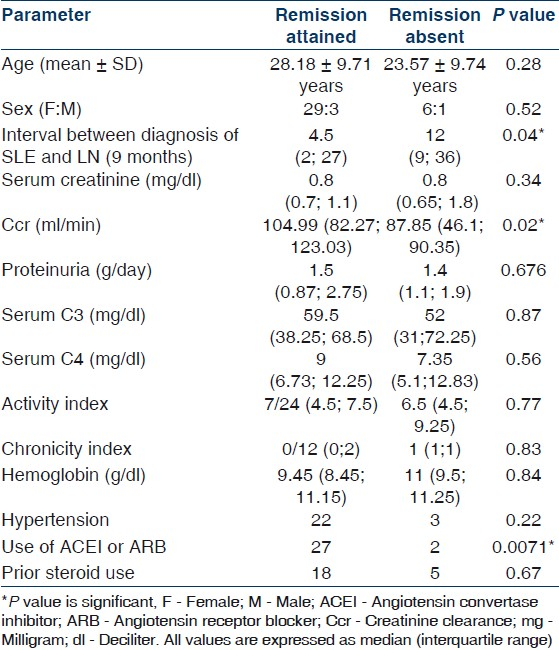
Chronic renal insufficiency (as defined under outcome measures and definitions) developed in five patients. The median interval before the development was 36 months (range 12- 72 months). Only one of these patients was dialysis dependent at the time of last follow-up visit [Figure 2].
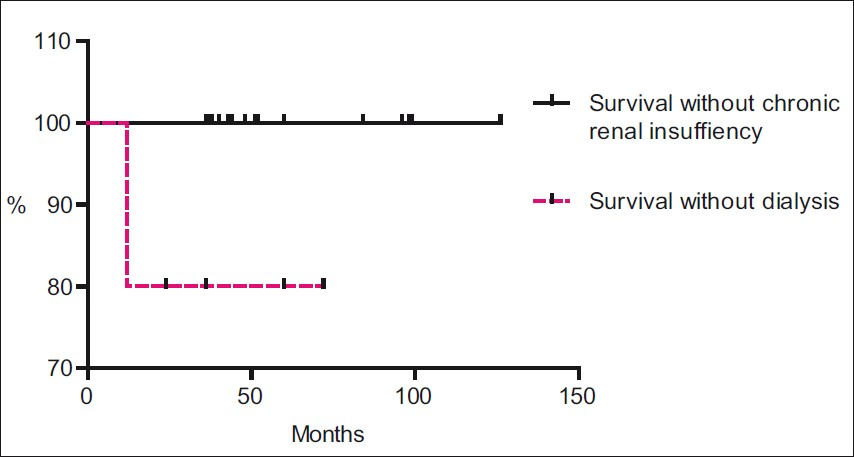
- Survival without doubling of serum creatinine and survival without dialysis
The risk of doubling of serum creatinine correlated with four factors [Table 3]. The most significant factor responsible for progression to chronic renal insufficiency was a lack of achievement of either a complete or partial remission (P<0.0001) [Figure 3]. A low eGFR at presentation favored a progression to chronic renal insufficiency (P=0.035). This lower eGFR might be responsible for lower proteinuria (P=0.01) in patients who progressed to chronic renal insufficiency. Nephritic renal flares were also significantly more common in patients who progressed to chronic renal insufficiency (P=0.0349).

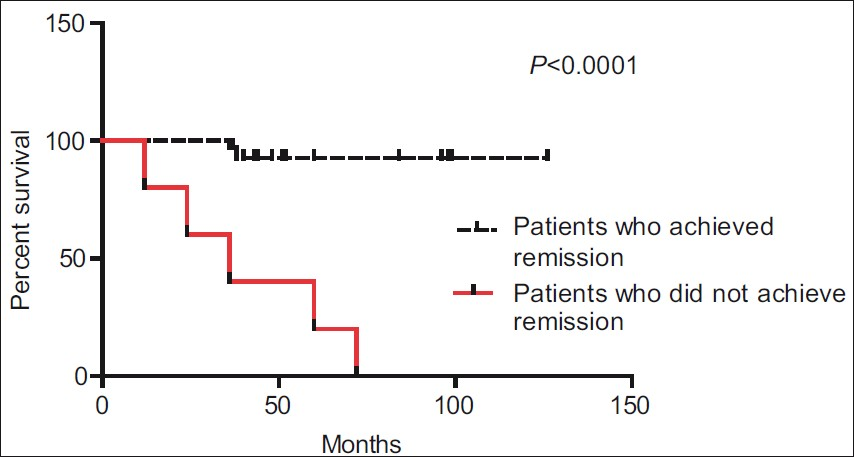
- Survival without doubling of serum creatinine in patients who achieved and who did not achieve remission
Following the completion of cyclophosphamide pulses, nine patients received MMF and the rest received AZA. One patient on MMF and four patients on AZA experienced a doubling of serum creatinine. The progression to chronic renal insufficiency was less common with MMF than with AZA (11.1% vs 13.3%) though this was not statistically significant (P=0.98). At the time of last follow up, the immunosuppressive drugs could be totally withdrawn for three of the 34 patients who did not experience a doubling of serum creatinine.
Adverse events
Nausea and vomiting were nearly universal with infusion of cyclophosphamide. The significant adverse events recorded were avascular necrosis of femur head in one patient, septic arthritis in one patient, pulmonary tuberculosis in one patient, amenorrhea in one patient, Herpes zoster in three patients, psychosis due to steroids was experienced by two patients, gluteal abscess in one patient and cataract in two patients. Two of the patients also developed diabetes mellitus at fifth and eighteenth month of therapy. Leucopenia necessitating either dose reduction or withdrawal of drugs was not recorded. Hemorrhagic cystitis was not seen in our patients.
Discussion
The outcome of lupus nephritis has improved since the introduction of cyclophosphamide. Still, depending on how it is defined and the ethnicity of the subjects included,[6] a significant proportion of patients with lupus nephritis do not achieve complete remission despite treatment with cyclophosphamide.
The mean age of subjects in our study was similar to the subjects who participated in the NIH study by Gourley et al.[7] The rate of complete and partial remission achieved in our study was similar to that achieved by Illei et al,(50.34 and 13.1% respectively)[8] but higher than that achieved in other studies[910] [Table 4]. A higher remission rate of 82% was achieved by Moroni G et al, but this was due to the use of oral cyclophosphamide with a higher cumulative dose[11] [Table 4]. A much higher remission rate (78%) was achieved with a longer duration of treatment by Ioannidis et al,[12] [Table 4]. A good remission rate in our study might have been significantly contributed by the early initiation of therapy. This is in contrast to other studies where a longer interval before the initiation of therapy favored a higher remission.[11] This contradictory finding may be due to the presence of a milder disease and a predominant Caucasian population in them. Comparing remission rates between studies of lupus nephritis is also limited by the varying definitions used to define remission. For instance, proteinuria of less than 1 g considered to be suggestive of remission by Gourley et al,[7] in the NIH studies is much lesser stringent than the criteria proposed by the Renal Subcommittee of Renal insufficiency of the American College of Rheumatology which we considered in our study.[5]

The other factors which assumed significance in predicting remission were a higher eGFR and concurrent use of ACE inhibitors [Table 2]. These factors were not found to be predictive by others.[10] Though serum creatinine was found to be predictive by Illei GG[8] and Moroni G[11] we did not find it to be predictive. It is known that serum creatinine lags the changes in GFR and hence may not be an early and accurate predictor in instances when therapy is initiated early as was done in our study. Though histological indices were found to be predictive of inducing remission by some,[6] this was not the case in our study [Table 2]. Similarly the histological indices (AI and CI) were not predictive of remission in studies which excluded patients with significant chronicity in the initial biopsy.[11]
The life expectancy of patients suffering with lupus nephritis has improved from a dismal 44% in the 1950s at 5 years[13] to 82% at 15 years in the 1990s.[14] There are few studies involving predominantly subjects from the Indian subcontinent which have looked into the outcome beyond just inducing remission.[4] Patients recruited into our study had a median follow up of 38 months (range being 36 to 126 months) and all of them were surviving at the end of the study period. Five patients had a doubling of serum creatinine and one of them was dialysis dependent. The improved outcome may have been definitely influenced by the use of cyclophosphamide in the early maintenance phase. Though some studies did not favor cyclophosphamide in the maintenance phase, there were limitations to their claims. The rate of infections was much higher than expected and it would be unfair to attribute all the infections solely to cyclophosphamide.[15] Another important limitation was the usage of cyclophosphamide in lower dosage than it would be normally used.[15]
Our study is a confirmation of the finding that lack of achievement of remission was significantly associated with the risk of developing renal insufficiency. Similar observations were also made by Moroni[12] and Houssiau et al.[16] Since all the patients in our study were treated by the same protocol, there can be little doubt that a low Ccr can be a significant factor that can predict the early progression to CKD. The adverse impact of nephritic renal flares is well known and findings similar to us were also demonstrated by others.[1718]
The impact of a higher chronicity index on histology favoring a progression to CKD was demonstrated by some,[1920] but not by others.[21] There was little chance for us to evaluate this aspect as we excluded patients with a high chronicity index. This exclusion was essential in our study so as to eliminate chronicity as a confounding factor when evaluating the role of cyclophosphamide.
In summary, therapy with cyclophosphamide, if initiated early, could be effective in inducing remission and could prevent the progression to CKD in Class IV lupus nephritis. The adverse effect profile is acceptable if the target dose is not exceeded. Attainment of remission either complete or partial has a significant impact on preventing the progression to CKD. The improved remission rates with ACE-i (not reported in previous studies) might be because of proteinuria reduction that such drugs produce. A study with a longer duration is necessary to demonstrate the benefit or a lack of it regarding the role of MMF in the maintenance phase.
Source of Support: Nil
Conflict of Interest: None declared.
References
- How did cyclophosphamide become the drug of choice for lupus nephritis? Nephrol Dial Transplant. 2009;24:381-4.
- [Google Scholar]
- Cytotoxic therapies of lupus nephritis: Recent developments. Nephrol Dial Transplant. 2002;17:955-7.
- [Google Scholar]
- Pulse cyclophosphamide in severe lupus nephritis: Southern Indian Experience. Saudi J Kidney Dis Transpl. 2010;21:372-8.
- [Google Scholar]
- Renal Disease Subcommittee of the American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria. The American College of Rheumatology response criteria for proliferative and membranous renal disease in systemic lupus erythematosus clinical trials. Arthritis Rheum. 2006;54:421-32.
- [Google Scholar]
- Severe lupus nephritis: Racial differences in presentation and outcome. J Am Soc Nephrol. 2007;18:244-54.
- [Google Scholar]
- Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis: A randomized, controlled trial. Ann Intern Med. 1996;125:549-57.
- [Google Scholar]
- Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy. Arthritis Rheumat. 2002;46:995-1002.
- [Google Scholar]
- Renal flares in 91 SLE patients diffuse proliferative lupus nephritis. Kidney Int. 2002;61:1502-9.
- [Google Scholar]
- Factors predictive of severe lupus nephritis.Lupus nephritis collaborative study group. Am J kidney Dis. 2000;35:904-14.
- [Google Scholar]
- Remission, relapse and remission of proliferative lupus nephritis treated with cyclophosphamide. Kidney Int. 2000;57:258-64.
- [Google Scholar]
- The long term outcome of 93 patients with proliferative nephritis. Nephrol Dial Transplant. 2007;22:2531-9.
- [Google Scholar]
- Long-term efficacy of azathioprine treatment for proliferative lupus nephritis. Rheumatology. 2000;39:969-74.
- [Google Scholar]
- Sequential therapies for proliferative lupus nephritis. N Engl J Med. 2004;350:971-80.
- [Google Scholar]
- Immunosuppressive therapy in lupus nephritis: The Euro-Lupus Nephritis trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46:2121-31.
- [Google Scholar]
- “Nephritic flares” are predictors of bad long-term renal outcome in lupus nephritis. Kidney Int. 1996;50:2047-53.
- [Google Scholar]
- Renal flares in 91 SLE patients with diffuse proliferative glomerulonephritis. Kidney Int. 2002;61:1502-9.
- [Google Scholar]
- Longterm follow-up of patients with lupus nephritis.A study based on the classification of the World Health Organization. Am J Med. 1987;83:877-85.
- [Google Scholar]
- Predicting renal outcomes in severe lupus nephritis: Contributions of clinical and histologic data. Kidney Int. 1994;45:544-50.
- [Google Scholar]
- Predictive value of renal pathology in diffuse proliferative lupus glomerulonephritis. Lupus Nephritis Collaborative Study Group. Kidney Int. 1989;36:891-6.
- [Google Scholar]







