Translate this page into:
Urinary indices during relapse of childhood nephrotic syndrome
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Sodium retention is the hallmark of idiopathic nephrotic syndrome (INS). Sodium retention could be secondary to activation of renin–angiotensin–aldosterone axis or due to an intrinsic activation of Na+K+ ATPase in the cortical collecting duct. Urine potassium/urine potassium + urine sodium (UK+/UK+ + UNa+) is a surrogate marker for aldosterone activity and can be useful in differentiating primary sodium retention from secondary sodium retention in children with INS. This was a cross-sectional study of children with INS, presenting to our center from June 2007 to June 2008. Children were categorized into those with steroid responsive and steroid nonresponsive nephrotic syndrome. One hundred and thirty-four children with nephrotic syndrome were analyzed. The FeNa+ was significantly lower during relapse than in remission but no such difference was observed with UK+/UK+ + UNa+. The values of FeNa+ and UK+/UK+ + UNa+ across various categories of nephrotic syndrome were similar. Correlating FeNa+ and UK+/UK+ + UNa+ with cut-off of 0.5 and 60%, respectively, we found 50% of steroid responsive children and 36% of steroid nonresponders having a corresponding UK+/UK+ + UNa+ of <60% along with low FeNa+ of <0.5%, favoring primary sodium retention. Urinary indices did not vary with the type of steroid response. In early relapse, the urinary indices revealed an overlap of both primary and secondary sodium retention in most stable edematous children with nephrotic syndrome.
Keywords
Aldosterone bioactivity
fractional excretion of sodium
nephrotic syndrome
sodium retention
urinary indices
Introduction
Renal sodium retention results from enhanced sodium reabsorption along the cortical collecting ducts and from blunted response of medullary collecting ducts to natriuretic response to atrial natriuretic peptide. The pathophysiology has been well described in children.[12] The traditional concept of sodium retention attributed to hypovolemia and activation of the renin–angiotensin–aldosterone system (secondary sodium retention) has been challenged in the recent years.[3–5] There is growing evidence of increased Na+K+ ATPase activity leading to sodium retention (primary retention) in the cortical collecting duct in nephrotic syndrome.[36] Secondary sodium retention occurs if proteinuria is severe enough to cause hypovolemia, seen only in a minority of adults. In contrast, children with nephrotic syndrome often display severe proteinuria, thus being more prone for secondary sodium retention.[78]
Children in early relapse may pass through a stage of incipient proteinuria and edema forming state with or without hypovolemia.[1] Clinical signs of hypovolemia are characterized by tachycardia, prolonged capillary filling rate and hypotension, stimulated by sodium retaining hormones and avid sodium retention in the edema forming state of a relapse. These patients exhibit clinical as well as laboratory evidence favoring secondary sodium retention. Functional hypovolemia may not present with clinical features of hypovolemia and results due to increase in vasoactive hormones with underlying normal blood volume. This discrepancy is probably related to efficient mechanisms aiming at maintaining blood volume.[1]
At the onset of a relapse, in a phase of incipient proteinuria, there is sodium retention with features of stimulated renin production, such as increased aldosterone bioactivity indicated by the enhanced Na+/K+ exchange measured by the index: urine potassium/urine potassium + urine sodium (UK+/UK+ + UNa+). During edema formation, without signs of hypovolemia, edema is not related to stimulated vasoactive hormones and secondary sodium retention, but suggests a primary tubular defect.[1] Early phase of full-blown nephrosis in children can present both with and without hypovolemic symptoms.[2]
There is an evidence that collecting duct Na+K+ ATPase activity correlates inversely with urinary sodium excretion (expressed as fractional excretion of sodium, FeNa+) in rats with nephrotic syndrome in addition to other mechanisms like proteolytic cleavage of epithelial sodium channel (ENaC) by proteases.[34] However, the measurement of FeNa+ alone cannot differentiate between primary and secondary sodium retention. Therefore, urinary indices which include urinary sodium and potassium values were correlated with serum aldosterone to differentiate primary from secondary sodium retention. Among them, indices like transtubular potassium gradient (TTKG), UK+/UNa+ had variable correlation with serum aldosterone, with the highest correlation (r = 0.758) achieved with UK+/UK+ + UNa+ index. UK+/UK+ + UNa+ ratio has been used as a marker for aldosterone activity with the assumption that Na+/K+ exchange occurs in the cortical collecting duct and is stimulated by aldosterone in hypovolemic patients with nephrotic syndrome.[9] This index has an advantage over TTKG which is dependent on osmolality or tonicity of urine.[10]
In patients with relapse, FeNa+ < 0.5% and UK+/UK ++ UNa+ < 60% would favor primary sodium retention, whereas FeNa+ < 0.5% and UK+/UK+ + UNa+ > 60% is associated with secondary sodium retention.[15]
In clinical practice, it is challenging to have supportive evidence of the type of sodium retention to predict the blood volume status during edema formation. Clinical assessment of underlying volume status is not accurate and is unreliable in the presence of edema. Hormonal assays and central venous pressure monitoring are not practical in every child. Surrogate markers like urinary indices can be used to evaluate the type of sodium retention. These indices would be useful to guide the clinician on using diuretics for management of edema.
The objectives of the study were to study the profile of urinary indices in children in early edema phase of relapse and to study the spectrum of urinary indices in various categories of nephrotic syndrome, based on the response to steroids.
Materials and Methods
This was a cross-sectional study involving children with idiopathic nephrotic syndrome (INS), presenting to the pediatric nephrology clinic from June 2007 to June 2008.
Inclusion criteria
a) Children with nephrotic syndrome, aged 1–12 years, following up at our center for at least 6 months; b) children in remission for 4 weeks without steroids or immunosuppressants; c) children in relapse for <2 weeks with edema; and d) steroid resistant (nonresponders) children in relapse.
Exclusion criteria
Children with systemic illness (fever, pneumonia, peritonitis, meningitis, urinary infection), hypovolemia (tachycardia, prolonged capillary filling rate, hypotension), fluid overload (respiratory distress, basal creptiations, hepatomegaly and raised jugular venous pulse); children receiving diuretics or immunosuppressant in the last 2 weeks, other than steroids; children with dyselectrolytemia (serum sodium < 135 or > 145 meq/l, serum potassium < 3.5 or > 5.5 meq/l), hypertension (blood pressures > 95th centile for age and renal dysfunction) (serum creatinine > 1 mg/dl) and secondary nephrotic syndrome (secondary to systemic diseases or drugs) were excluded.
After obtaining an informed consent from parents for the study and an approval from the ethical committee of the institution, children were examined and the demographic profile was documented. They were categorized as per National Kidney Foundation consensus[11] guidelines into four groups as follows:
Group 1: remission
Group 2: relapse. The children with relapse were further grouped according to clinical course as
Group 3: steroid responders:
-
infrequent relapses (IFR) with ≤2 relapses in 6 months or ≤3 relapses in a year
-
frequent relapses (FRNS) with ≥2 relapses in 6 months or ≥3 relapses in a year
Group 4: steroid nonresponders (SNR) comprising those who have not attained remission with 4 weeks of daily steroid therapy at 2 mg/kg/day.
All children were allowed to have normal diet salt and fluid intake.
A fresh second morning spot urine sample was obtained for albumin, sodium, potassium and creatinine estimation. A simultaneous serum sample was collected to measure sodium, potassium, albumin and creatinine values using a Dade Behring RXL Max Analyzer.
The following urinary indices were calculated
-
FeNa+ (%): (urine sodium × serum creatinine/serum sodium × urine creatinine) × 100
-
urine potassium (UK+)/urine sodium (UNa+) + urine potassium × 100 (%).
Primary sodium retention was defined as FeNa+ <0.5% and UK+/UK+ +UNa+ <60%
Secondary sodium retention was defined as FeNa+ <0.5% and UK+/UK+ + UNa+ >60%[19]
Categorical data were expressed as proportions (percentage) and continuous data as median with IQR (interquartile range 25th, 75th percentile).
The association between the urinary indices was examined using scatter plot. Parameters were compared between groups with Mann Whitney U test. All analyses were performed with SPSS, version 13.
Results
One hundred and forty-seven children with nephrotic syndrome were recruited and 134 children met the criteria and were included in the study. Thirty-eight children were in remission and 96 were in relapse. Among the children in relapse, 53 had infrequent relapses, 32 had frequent relapses and 11 were steroid nonresponders [Figure 1]. The demographic data are shown in Table 1. The mean duration of edema in the steroid responders was 7.5±3 days and it was 12±2 days in steroid resistant children. Ten out of the 32 children with a frequent relapsing course and 7 of the 11 steroid resistant children were on <1 mg/kg/day of oral steroids for 4 weeks. None of the patients were on angiotensin converting enzyme inhibitors or angiotensin receptor blockers. The mean values of serum sodium, potassium, creatinine, albumin and urine protein creatinine ratios in the three categories are depicted in Table 2. The histopathology of the steroid resistant children revealed minimal change disease in seven and mesangial hypercellularity with immunoflorescent IgM deposits in the mesangium in four patients.
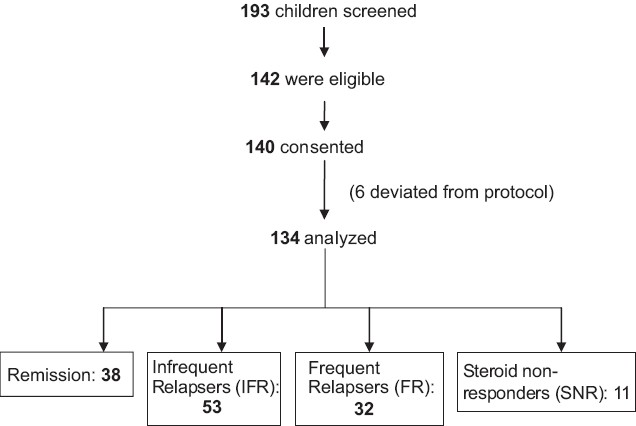
- Patient recruitment


Remission versus relapse
The median FeNa+ was significantly lower in the relapse group (0.24%) as compared to the remission group (0.45%) (P = 0.002) [Figure 2]. There was no significant difference in the median UK+/UK+ + UNa+ index between the two groups (P=0.86) [Figure 3].

- FeNa+ in remission and relapse, expressed as median with confidence interval

- UK+/UK+ + UNa+ in remission and relapse, expressed as median with confidence interval
There was no significant difference in the median FeNa+ and UK+/UK+ + UNa+ in steroid responders (frequent relapsing, infrequent relapsing groups) and steroid nonresponders [Figures 4 and 5]. Among the steroid responders and nonresponders, sodium retention (FeNa+ < 0.5%) was seen in 67% and 73%, respectively.

- FeNa+ in steroid responders (IFR, FRNS) and nonresponders (SNR)

- UK+/UK+ + UNa+ in responders and nonresponders
FeNa+ and UK+/UK+ + UNa+ in steroid responders and steroid nonresponders
Correlating FeNa+ and UK+/UK+ + UNa+ with cut-off of 0.5 and 60%, respectively, we found 50% of steroid responsive children and 36% of steroid nonresponders having a corresponding UK+/UK+ + UNa+ of <60% along with low FeNa+ of <0.5%, favoring primary sodium retention [Figure 6]. It was observed that 32% of responders and 27% of nonresponders had an FeNa+ of >0.5% and UK+/UK+ + UNa+ <60%, suggesting no significant sodium retention and no secondary hyperaldosteronism.

- Cross-tabulation of steroid responders and nonresponders with FeNa+ <0.5% and UK+/UK+ + UNa+ <60% (50 and 36%, respectively) and FeNa+ <0.5% and UK+/UK+ + UNa+ >60% (17 and 36%, respectively)
Discussion
There is sodium retention during a relapse of nephrotic syndrome and this can be due to primary or secondary sodium retention.[112–15] An FeNa+ of <0.5% and UK+/UK+ + UNa+ ratio of <60% points towards primary sodium retention.[15]
Previous work done by Donckerwolcke et al,[29] reveals normal renal sodium handling and vasoactive hormones in remission, with an average FeNa+ of 0.7% and UK+/UK+ + UNa+ of 31%. Sodium retention was noticed early at onset of incipient proteinuria during a relapse with an average FeNa+ of 0.2% and UK+/UK+ + UNa+ of 60%, which was similarly noticed in our study. In patients retaining sodium (FeNa+ < 0.5%) with increased aldosterone levels, a corresponding UK+/UK+ + UNa+ ratio >30% and most often higher than 60% was observed by Donckerwolcke et al.[9] We would like to highlight the fact that we have assessed the urinary indices in clinically stable children without hypovolemic symptoms in relapse and found an overlap of urinary indices, suggesting either primary or secondary sodium retention.
Sodium retention in active edema forming states was shown not to be related to plasma aldosterone concentration, favoring an intrarenal mechanism of sodium retention.[16] Vande Walle et al,[2] described three presentations of children in early relapse. The groups consisted of patients with (a) incipient proteinuria, sodium retention and slightly elevated aldosterone; (b) symptoms of hypovolemia, edema, sodium retention and elevated rennin and aldosterone; and (c) edema, no hypovolemia, no active sodium retention and normal plasma hormones. Our study reveals sodium retention in early relapse which was not always associated with an increased UK+/UK+ + UNa+ ratio that would favor underlying hyperaldosteronism. We observed that the children with no symptoms of hypovolemia in the early phase of relapse could present both with and without active sodium retention. Those who retained sodium had associated features of either primary or secondary sodium retention. This finding may be explained by the fact that our patients could have been in the incipient proteinuric phase or edema forming phase of a relapse and have variations in the urinary indices.
There are no studies evaluating the spectrum of urinary indices in children with regard to clinical course or response to steroid therapy.
We found no significant difference in the extent of sodium retention with regard to steroid response. Renal sodium handling has not been found to be different in minimal change disease and nephrotic syndrome with different histological lesions.[17]
The possibility of utility of renin sodium profiling in predicting steroid responsiveness was proposed by Meltzer et al,[18] in his report which showed that patients with high renin values were steroid responsive and low renin values correlated with steroid unresponsiveness.
In our study, the sensitivity and specificity of FeNa < 0.5% with UK+/UK++ U Na+ < 60% to predict steroid nonresponders was very low (36 and 50%, respectively).
Our study had some limitations. Evaluating the indices in various phases of clinical course (remission, relapse, clinical hypovolemia) in the same patient, who could act as their own controls, will add more information on the pathophysiology. Correlation with serum aldosterone assay with age-matched controls can throw more light on this concept.
Conclusion
In our study, the profile of urinary indices did not vary with the type of steroid response. In early relapse, the urinary indices in most stable edematous children with nephrotic syndrome reveal an overlap of both primary and secondary sodium retention. Using diuretics to reduce edema without evaluating the underlying blood volume status may be a challenge for the clinicians. Use of diuretics would be safe in the edema forming phase of relapse only if secondary sodium retention is excluded.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Pathogenesis of edema formation in the nephrotic syndrome. Pediatr Nephrol. 2001;16:281-93.
- [Google Scholar]
- Volume regulation in children with early relapse of minimal change nephrosis with or without hypovolemic symptoms. Lancet. 1995;346:148-52.
- [Google Scholar]
- Molecular mechanism of edema formation in nephrotic syndrome: Therapeutic implications. Pediatr Nephrol. 2007;22:1983-90.
- [Google Scholar]
- Mechanisms of edema in nephrotic syndrome: Old theories and new ideas. Nephrol Dial Transplant. 2003;183:454-6.
- [Google Scholar]
- Is edema in minimal change disease of childhood really hypovolemic. Int Urol Nephrol. 2008;40:757-61.
- [Google Scholar]
- Collecting duct Na/K ATPase activity is correlated with urinary sodium excretion in rat nephrotic syndromes. J Am Soc Nephrol. 2000;11:604-15.
- [Google Scholar]
- Observations on edema formation in the nephrotic syndrome in adults with minimal lesions. Am J Med. 1979;67:378-84.
- [Google Scholar]
- Blood volume, colloid oncotic pressure and T cell ratio in children with nephrotic syndrome. Kidney Int. 1996;49:1471-7.
- [Google Scholar]
- Distal nephron Na –K exchange in children with nephrotic syndrome. Clin Neph. 2003;59:259-65.
- [Google Scholar]
- Development of a test to evaluate the transtubular gradient in cortical collecting duct in vivo. Miner Electrolyte Metab. 1986;12:226-33.
- [Google Scholar]
- Evaluation and management of protein uria and nephrotic syndrome in children: Recommendations from a Pediatric Nephrology Panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection and elimination. Pediatrics. 2000;105:1242-8.
- [Google Scholar]
- Na, K ATPase activity and hormones in single nephron segments from nephrotic rats. Clin Sci. 2003;80:599-604.
- [Google Scholar]
- Renal sodium handling in nephrotic relapse: Relation to hypovolemic symptoms. Nephrol Dial Transpl. 1996;11:2202-8.
- [Google Scholar]
- Pathogenesis of edema in nephrotic syndrome. In: Seldin DW, Giebisch, eds. The regulation of sodium and chloride balance. New York: Raven; 1990. p. :321-51.
- [Google Scholar]
- Renal sodium handling in minimal change nephrotic syndrome. Arch Dis Child. 1984;59:825-30.
- [Google Scholar]
- Pathophysiology of edema formation in children with nephrotic syndrome not due to minimal change disease. J Am Soc Nephrol. 1999;10:323-31.
- [Google Scholar]
- Nephrotic syndrome: Vasoconstriction and hypervolemic types indicated by renin-sodium profiling. Annals of Int Med. 1979;91:688-96.
- [Google Scholar]







