Translate this page into:
Human leukocyte antigen antibody incompatible renal transplantation
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anti-human leukocyte antigen (HLA) antibodies are recognized as an important problem in organ transplant recipients. This is because antibodies formed against a graft months or years after implantations are the major cause of late allograft failure, and also because protocols allow the transplantation of some grafts across pre-formed HLA antibodies. Advances in our understanding of anti-HLA antibody- mediated rejection (AMR) have occurred because of a better understanding of the histological findings during AMR; more sensitive and specific methods to measure anti-HLA antibodies; and through clinical investigation of patients transplanted across an HLA barrier. Despite advances in therapy and investigation, AMR remains a major problem and treatment protocols often fail to treat it successfully. This review aims to describe the issues in each of these areas and to suggest how clinicians may be able to improve the management of patients with anti-HLA antibodies.
Keywords
Antibody
incompatible
kidney
transplantation
Introduction
Antibody incompatible transplantation (AiT) is defined as transplantation across an Human Leukocyte Antigen (HLA) antibody barrier, with defined donor-specific antibody being present at the time of transplantation or at the initiation of pre-transplant conditioning. In recent times, there has been a steady increase in antibody incompatible transplantation. This is because many protocols involving plasmapheresis have shown reasonable success in short and medium term outcomes.[1] Also, newer assays have made it possible for identification of previously undetectable levels of donor-specific antibodies (DSA).[2] The main advances of the last two decades are the ability to identify DSA with a sensitive microbead assay, and to transplant with some early success across all but the highest levels of DSA.[3] These transplants have an increased risk of acute antibody-mediated rejection (AMR).
Hyperacute Rejection
Antibody-mediated hyperacute rejection was recognized in late 1960s. Williams reported that DSA against HLA can cause hyperacute rejection in clinical transplantation.[4] This was followed in 1969 by the development of a cross-match technique that could be performed reproducibly and had a good correlation with clinical outcome.[5] Hyperacute rejection has now been virtually eliminated, but little progress has been made in the understanding of lesser degrees of AMR.
Histology of Antibody-Mediated Rejection
Clinical interest in AMR resurfaced in the early 1980s when Halloran and colleagues[67] described the pathological features of acute AMR using light electron microscopy. They showed that in acute AMR it was generally not possible to demonstrate the presence of antibody in the graft, so the diagnosis relied upon indirect markers of antibody-mediated rejection. These could be a picture of acute tubular necrosis, or varying degrees of cellular infiltration into glomeruli or peritubular capillaries with an associated inflammatory response including glomerulitis and interstitial hemorrhage. These findings still form the basis of the histologic classification of AMR, which has been refined in the Banff classification.[8] Recent studies have shown that the cellular infiltrate in acute AMR does contain macrophages and neutrophils, as originally described, but is also characterized by a high proportion of T cells.[9] Indeed, the T cell signature of cellular rejection (T cell mediated rejection) is the same as that of acute AMR.[10] The identification of C4d as a pathological marker for AMR in clinical transplants,[1112] is an important development, though AMR may occur in the absence of C4d staining[9] [Figure 1]. Further refinement of the BANFF classification is likely to place more stress on cellular changes and less importance on C4d staining. A method to detect antibody in histologic sections and apply this to clinical diagnosis is awaited.
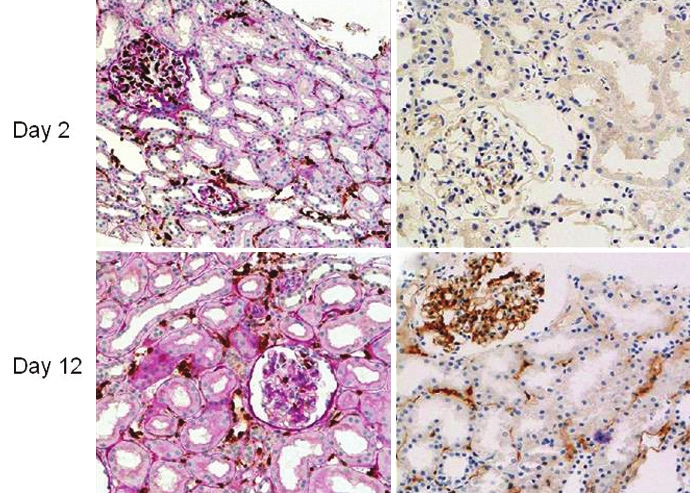
- C4d staining may not be apparent at the onset of antibody-mediated rejection
AMR is a consequence of the interaction of vascular endothelium of the graft with anti-donor antibodies, though there is still speculation as to whether an additional direct T cell mediated response is important in some patients. Endothelial cells play an important role in movement of molecules between the intravascular and extravascular compartments. DSA binds with endothelial cells and cause complement activation, resulting in cell death and subsequent ischemic injury.[13] The negatively charged heparin sulfate on the endothelial surface repels negatively charged plasma proteins like albumin and coagulation factors.[14] The ischemic damage to the endothelial cells by the DSA results in the formation of gaps between the cells due to the loss of electronegativity. This causes sub-endothelial matrix to bind with plasma coagulation factors resulting in vascular thrombosis.[15]
After the acute phase, the peritubular capillaritis is thought to progress into multi-layering of basement membrane.[16] There is also the development of transplant glomerulopathy (TG) which is increasingly recognized as a manifestation of chronic antibody-mediated injury. TG is characterized by double contour of glomerular and peritubular capillary basement membranes and deposition of C4d in peritubular capillaries on the biopsy, and proteinuria.[17] More recently, pathologic, physiologic or molecular evidence of endothelial disturbance in the absence of demonstrable C4d deposits has been correlated with chronic graft failure.[18] If TG is seen on a biopsy, care should also be also taken to document the extent of ongoing peri-tubular capillaritis, since it is possible that the cellular infiltration may be more amenable to therapy than glomerular damage.
Detection of Human Leukocyte Antigen Antibodies
Tests to measure HLA antibodies have improved in sensitivity and specificity over the years. However, there is still some way to go before clinically relevant antibodies can be measured accurately, especially in patients who have a functioning graft where DSA may be absorbed onto the graft and affect the blood levels of DSA.
Complement-dependent cytotoxicity (CDC) crossmatching was pioneered by Terasaki and colleagues in the 1960s.[5] It seeks to identify clinically significant donor-specific HLA antibody-mediated responses for a given recipient. Lymphocytes from the donor are isolated and separated into T and B cells. Serum from the recipient is mixed with the lymphocytes and complement (rabbit serum) is then added. If donor-specific antibody is present and binds to donor cells, the complement cascade will be activated via the classical pathway resulting in lysis of the lymphocytes. The read-out of the test is the percentage of dead cells relative to live cells as determined by microscopy. The result can be scored on the percentage of dead cells, with 0 correlating to no dead cells; scores of 2, 4, and 6 represent increasing levels of lysis. To determine the strength of the reaction the cross-match can be repeated using serial doubling dilutions of the recipient serum (often known as a ‘titered crossmatch’). In this way, dilutions are usually performed to 1 in 2, 4, 8, 16, 32, 64, and so on. With antibody at a low level, a single dilution may be enough to render the cross-match result negative. This may also give an indication as to the likelihood that a negative cross-match could be achieved with a desensitization protocol. The basic CDC cross-match can be enhanced by the addition of antihuman globulin (AHG). This result in multiple AHG molecules binding to each DSA attached to the donor cells thereby amplifying the total number of Fc receptors available for interaction with complement. This technique increases the sensitivity of the CDC cross-match by increasing the complement activation and cell lysis.[19]
The CDC cross-match can be false-positive because of a technical issue or because of the presence of autoantibodies present in the recipient serum. Autoantibodies are generally IgM rather than IgG antibodies. To establish if autoantibodies are responsible for the result, an auto-crossmatch can be performed, where the recipient serum is cross-matched against their own lymphocytes. Also, the original cross-match could be repeated with the addition of the agent Dithiothreitol (DTT). DTT reduces the disulfide bonds in IgM thereby preventing IgM antibodies from generating a positive result. It is also possible to have a negative cross-match in the presence of a DSA, when the antibody titer is too low to cause complement activation or when the antibody does not activate complement or when the antigen for which the antibody is specific is expressed only at very low levels on the donor's lymphocytes.
There are differences in interpretation of B cell and T cell cross-match. T cells do not express HLA class II so the result of a T-cell cross-match generally reflects antibodies to HLA class I only. B cells, on the other hand, express both HLA class I and II, so a positive B-cell cross-match may be due to antibodies directed against HLA class I or II or both. Therefore, if both T and B cell cross-matches are positive, it could be either due to class I DSA or a mixture of class I and II. If it is a negative T-cell but positive B-cell cross-match it could be due to only class II DSA or a low level of class I DSA. This is because B cells express higher levels of HLA class I than T cells.[20]
Flow cytometric cross-matching is more sensitive than CDC cross-matching. It is performed by mixing donor cells and recipient serum and incubating them with fluorescein-labeled antibodies against human IgG (antihuman IgG), which was first described in 1983.[21] This fluorescein-labeled antibody will bind to all the IgG antibodies in the recipient serum. If there is DSA in the serum then it binds to the donor lymphocytes and this will be detectable by flow cytometry. For B cell cross-match adding PE-conjugated CD-19 and for T cell cross-match adding PE-conjugated CD3 was carried out. The readout is the comparison of the test result over that for a negative control AB serum. This readout can be expressed in a number of different ways, which means that it is not possible to compare the results of FC cross-matching in different laboratories. Different readouts of test over control include relative median fluorescence, channel shifts, or the number of doubling dilutions of test serum required to render the test readout negative.
CDC and FC cross-matching require donor cells, which makes the test cumbersome to perform repeatedly and adds to variability of test result. Over the last decade, it has become increasingly feasible to purify HLA proteins, which gives the potential to measure HLA antibodies in a reliable sold phase assay. The best of these to date is the microbead assay, analyzed on the Luminex platform. Recipient serum potentially containing anti-HLA antibodies is added to a mixture of synthetic beads. Each test sample contains up to 96 types of bead, each of which can be separately identified by the Luminex platform. A different HLA protein is attached to each type of bead. We can thus identify many different antibodies, and compare them with the donor's HLA antigens, thus enabling a prediction of crossmatch result. The advantages of Luminex testing are not having false-positive reactions and being able to determine the correct antibody specificity. Luminex testing also has some limitations including variation in HLA density on beads; difficulty attaching purified HLA protein to the beads with any denaturing of antigenic binding sites; and providing a dynamic range that would allow conversion of the fluorescence readout to absolute antibody concentration.[22]
The presence of a DSA detected by Luminex with CDC negative crossmatch appears to have prognostic importance in terms of graft survival and acute rejection risk. We have shown that HLA antibody incompatible renal transplantation had a high success rate if the CDC crossmatch was negative. Death censored graft survival at 1, 3, and 5 years was 97.5%, 94.2%, and 80.4% in all DSA-positive patients. At five years, the death censored graft survival in the CDC crossmatch positive group was 54.3% [Figure 2]. In comparison, in the CDC negative crossmatch and DSA positive group the graft survival was 88.6%, which was statistically significant (P < 0.03). Also the five-year graft survival in the DSA negative group was 80.2%.[23]

- Death censored graft survival based on pre-treatment donor-specific antibodies levels
We had also previously shown that DSA either caused no rejection after HLA antibody incompatible transplantation, or rejection was resolved in the presence of DSA in the majority of cases, possibly due to accommodation.[24]
Management of Antibody-Mediated Rejection
Prevention and treatments for acute and chronic HLA antibody-mediated damage are not yet fully effective, but there is scope for considerable optimism. For example, in our series of HLA antibody incompatible transplants, the early response rate to therapy for acute AMR was greater than 95%. However, there are two main problems. First, treatment of acute AMR is far less effective when antibodies are present at a level that is strongly CDC positive, and second, some acute AMR progresses to a chronic phase with transplant glomerulopathy and eventual graft failure.
There are many different management protocols available for acute and chronic AMR. These include plasmapheresis (PP), intravenous immunoglobulin (IVIg), anti-thymocyte globulin (ATG), rituximab, splenectomy, bortezomib, and eculizumab in various combinations and dosage.
These different treatments have not been tested in appropriate randomized trials, so that their use is based on individual clinical preferences, which continue to differ widely between clinicians. This suggests that either the treatments are all effective, or that acute AMR may resolve irrespective of the intervention. Certainly, we have noted that in many cases with a sharp rise in DSA at about 10 days post-transplant and acute AMR, the graft recovers whereas DSA is still present, and then a few days later there may be dramatic fall in DSA levels that is not related to any particular therapy other than routine induction immunosuppression and high dose of methyl prednisolone. This apparent ability of the graft to recover function and for the DSA to disappear suddenly makes it easy for claims to be made for the efficacy of any individual treatment based on limited anecdotal experience.
An initial study demonstrated that protocols using multiple plasmapheresis treatments leads to more reproducible desensitization and lower humoral rejection rates when compared with a single high dose intravenous immunoglobulin (IVIG).[25] The Cedars-Sinai hospital which uses IVIg in high-immunological risk patients is associated with good one-year outcomes, adequate GFR, and a profound decrease in panel reactive antibodies, but a significant increase in allograft nephropathy.[26] However, in this center patient not responding to IVIg did not always proceed to transplant. The Mayo Clinic, in a less selected and higher risk patient group, found that high dose IVIg alone is inferior to plasmapheresis and IVIg and anti-CD20 as therapy for AMR.[27]
At the Johns Hopkins University, acute severe AMR has been treated with emergency splenectomy followed by plasmapheresis and IVIg. Five patients who experienced an acute deterioration in renal function and had a rise in donor-specific antibody within the first post-transplant week after desensitization, had undergone immediate splenectomy followed by plasmapheresis and IVIg resulting in return of allograft function within 48 h of the procedure.[28]
They also presented a single case in which eculizumab, a complement protein C5 antibody that inhibited the formation of the membrane attack complex (MAC), was used in combination with plasmapheresis and IVIg to salvage a kidney undergoing severe AMR. This resulted in a marked decrease in C5b-C9 (MAC) complex deposition in the kidney.[29] In a recent study published by Mayo clinic, they have shown that the incidence of AMR was 7.7% (2/26) in the eculizumab group compared to 41.2% (21/51) in the control group (P = 0.0031). Eculizumab also decreased AMR in patients who developed high levels of DSA early after transplantation that caused proximal complement activation. On one-year protocol biopsy, transplant glomerulopathy was found to be present in 6.7% (1/15) eculizumab-treated recipients and in 35.7% (15/42) of control patients (P = 0.044). Thus inhibition of terminal complement activation with eculizumab was thought to decrease the incidence of early AMR in sensitized renal transplant recipients.[30] Our experience has been that though a case can be made for a positive effect of eculizumab the drug was not completely successful in abrogating rejection in the presence of extremely high DSA levels.[31] In the absence of a “curative” drug for AMR, eculizumab may represent a useful addition to the nephrologist's toolbox.
The proteasome inhibitor, bortezomib, treatment might in theory be useful, as it is designed to kill plasma cells. Invitro studies indicate that it is capable of killing plasma cells and stopping HLA antibody secretion.[32] The Cincinnati center has most experience of bortezomib, reporting reversal of rejection and reduction in DSA levels.[33] However, a recently reported study did not show any significant decrease in DSA following bortezomib treatment in patients awaiting transplantation. In addition, as antiviral antibody levels remained stable following treatment, they concluded a lack of efficacy on long-lived plasma cells.[34] Despite this there remains the possibility that activated plasma cells post-transplant are more sensitive to bortezomib than those in steady- state tickover, and a randomized clinical trial is urgently required.
In our center, we have linked our therapeutic approach to histologic finding of T cell infiltration in acute AMR, and the mainstay of treatment is either OKT3 (previously) or polyclonal ATG (currently) which has shown early reversal of rejection in over 95% of cases.[23] We do not use IVIg, and have phased out post-transplant plasmapheresis as it is associated with increased complication rates, and is not able to control DSA levels effectively during periods of rapid synthesis.[35]
Implications for Clinical Practice
How should clinicians respond to their patients with HLA antibodies?
Everyone looking after long-term patients will encounter chronic AMR in a significant proportion of patients. This should be detected at an early stage by careful monitoring of renal function, and significant deterioration on renal function not explained by infection or structural problem should always be investigated by renal biopsy. DSA monitoring may be useful but as many patients have chronic AMR with DSA in their blood (presumably absorption of antibody onto the graft) it is not an useful screening tool. If chronic AMR is diagnosed, there is no treatment of proven usefulness. It would seem reasonable to increase exposure to steroids and azathioprine or mycophenolate, optimize other risk factors, like controlling proteinuria, blood pressure with ACE inhibitors, while awaiting the results of randomized studies.
The more pressing issue is whether to transplant patients who have DSA against a donor kidney, especially when this is available from a living donor. These transplants have only been performed for a few years, and there are few long term data on the outcomes. These data often come from single centers, and inevitably these may present the better end of the spectrum of outcomes. A UK national Registry of antibody incompatible transplantation is the first of its type in the world, and a large collaborative study in the USA has started to report at a conference on medium term outcomes.[36] One has to be cautious about the outcomes. Even at low levels of DSA, graft outcome may be compromised.[37] The early and medium term results of transplanting across CDC crossmatch seem poor, with about 50% of 5-year graft survival.[23] A particular issue in HLA AiT is that many patients who had previous failed grafts had renal failure for many years, and this exposes them to a high risk of death. In our center the 2-year mortality of HLA AiT is about 5%, but rises to 20% if higher risk patients are accepted.[38] Even though the mortality in our series was low, 25% of patients had unplanned admission to critical care.
It is suggested that transplantation across pre-formed HLA antibodies is not yet a routine practice, and should be performed as part of structured programs in specialist centers, as suggested in the British Transplantation Society and British Society for Histocompatibility and Immunogenetics guidelines.[3940] A laboratory supporting a program of AiT must have robust and accurate methods to distinguish HLA antibodies directed against the donor. AiT should not be performed if only CDC and not flow cytometry or Luminex is available to quantify HLA antibodies, even with prophylactic ATG. Research remains critical, both at the level of recruiting to available clinical trials and participating in laboratory research.
In conclusion, advances have been made in the understanding of and treatment of antibody-mediated rejection in the last decade, but the clinical outcomes are not yet satisfactory. To use the cricketing analogy, much more ‘net practice’ is required, but many of the tools to achieve selective elimination of HLA antibodies are emerging and the next generation should be competitive at ‘Premier League’ level.
Source of Support: Nil
Conflict of Interest: None declared
References
- Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582-9.
- [Google Scholar]
- Sensitization and sensitivity: Defining the unsensitized patient. Transplantation. 2000;69:1370-4.
- [Google Scholar]
- Prevention of hyperacute rejection by removal of antibodies to HLA immediately before renal transplantation. Lancet. 1996;348:1208-11.
- [Google Scholar]
- Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-9.
- [Google Scholar]
- The significance of the anti-class I antibody response. I. Clinical and pathologic features of anti-class I-mediated rejection. Transplantation. 1990;49:85-91.
- [Google Scholar]
- The significance of the anti-class I response. II. Clinical and pathologic features of renal transplants with anti-class I-like antibody. Transplantation. 1992;53:550-5.
- [Google Scholar]
- Banff ‘09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464-71.
- [Google Scholar]
- The histological development of acute antibody-mediated rejection in HLA antibody-incompatible renal transplantation. Nephrol Dial Transplant. 2010;25:1306-12.
- [Google Scholar]
- The role of B cells and alloantibody in the host response to human organ allografts. Immunol Rev. 2003;196:197-218.
- [Google Scholar]
- Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol. 1991;86:464-70.
- [Google Scholar]
- Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208-14.
- [Google Scholar]
- Release of heparan sulfate from endothelial cells.Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990;171:1363-8.
- [Google Scholar]
- Transient perturbation of endothelial integrity induced by natural antibodies and complement. J Exp Med. 1995;181:21-31.
- [Google Scholar]
- Thickening of the peritubular capillary basement membrane is a useful diagnostic marker of chronic rejection in renal allografts. Am J Transplant. 2007;7:923-9.
- [Google Scholar]
- Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312-23.
- [Google Scholar]
- Understanding crossmatch testing in organ transplantation: A case-based guide for the general nephrologist. Nephrology (Carlton). 2011;16:125-33.
- [Google Scholar]
- B peripheral lymphocytes express more HLA antigens than T peripheral lymphocytes. Transplantation. 1978;25:93-5.
- [Google Scholar]
- Flow cytometry analysis: A high technology crossmatch technique facilitating transplantation. Transplant Proc. 1983;15:1939-44.
- [Google Scholar]
- Naturally occurring interference in Luminex assays for HLA-specific antibodies: Characteristics and resolution. Hum Immunol. 2009;70:496-501.
- [Google Scholar]
- Human leukocyte antigen antibody-incompatible renal transplantation: Excellent medium-term outcomes with negative cytotoxic crossmatch. Transplantation. 2011;92:900-6.
- [Google Scholar]
- Blood levels of donor-specific human leukocyte antigen antibodies after renal transplantation: Resolution of rejection in the presence of circulating donor-specific antibody. Transplantation. 2007;84:876-84.
- [Google Scholar]
- A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6:346-51.
- [Google Scholar]
- Posttransplant prophylactic intravenous immunoglobulin in kidney transplant patients at high immunological risk: A pilot study. Am J Transplant. 2007;7:1185-92.
- [Google Scholar]
- Comparison of combination Plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant. 2009;9:1099-107.
- [Google Scholar]
- The utility of splenectomy as rescue treatment for severe acute antibody mediated rejection. Am J Transplant. 2007;7:842-6.
- [Google Scholar]
- The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9:231-5.
- [Google Scholar]
- Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405-13.
- [Google Scholar]
- C5b-9 inhibitor (eculizumab) for antibody-mediated rejection in renal transplantation. Indian J Transplant. 2011;1:6-8.
- [Google Scholar]
- Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201-9.
- [Google Scholar]
- Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754-61.
- [Google Scholar]
- Bortezomib as the sole post-renal transplantation desensitization agent does not decrease donor-specific anti-HLA antibodies. Am J Transplant. 2010;10:681-6.
- [Google Scholar]
- Rises and falls in donor-specific and third-party HLA antibody levels after antibody incompatible transplantation. Transplantation. 2009;87:882-8.
- [Google Scholar]
- Incompatible live-donor kidney transplantation in the United States: Results of a national survey. Clin J Am Soc Nephrol. 2011;6:2041-6.
- [Google Scholar]
- Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant. 2011;11:470-7.
- [Google Scholar]
- Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318-26.
- [Google Scholar]
- Antibody Incompatible Transplant Guidelines - British Transplantation Society Standards. 2011. Available from: http://www.bts.org.uk
- [Google Scholar]
- BSHI and BTS Guidelines for the Detection and Characterisation of Clinically Relevant Antibodies in Allotransplantation. 2011. British Transplantation Society Standards. Available from: http://www.bts.org.uk
- [Google Scholar]







