Translate this page into:
Revised guidelines on management of antenatal hydronephrosis
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Widespread antenatal screening has resulted in increased detection of anomalies of the kidneys and urinary tract. The present guidelines update the recommendations published in 2000. Antenatal hydronephrosis (ANH) is transient and resolves by the third trimester in almost one-half cases. The presence of oligohydramnios and additional renal or extrarenal anomalies suggests significant pathology. All patients with ANH should undergo postnatal ultrasonography; the intensity of subsequent evaluation depends on anteroposterior diameter (APD) of the renal pelvis and/or Society for Fetal Urology (SFU) grading. Patients with postnatal APD exceeding 10 mm and/or SFU grade 3-4 should be screened for upper or lower urinary tract obstruction and vesicoureteric reflux (VUR). Infants with VUR should receive antibiotic prophylaxis through the first year of life, and their parents counseled regarding the risk of urinary tract infections. The management of patients with pelviureteric junction or vesicoureteric junction obstruction depends on clinical features and results of sequential ultrasonography and radionuclide renography. Surgery is considered in patients with increasing renal pelvic APD and/or an obstructed renogram with differential renal function <35-40% or its subsequent decline. Further studies are necessary to clarify the role of prenatal intervention, frequency of follow-up investigations and indications for surgery in these patients.
Keywords
Pelviureteric junction obstruction
posterior urethral valves
renography
vesicoureteric reflux
Introduction
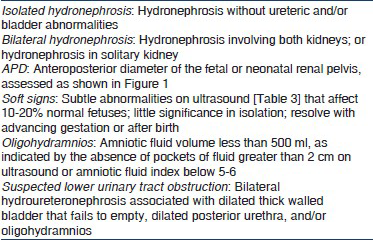
Ultrasound screening during pregnancy has resulted in increasing recognition of fetal hydronephrosis. Depending on diagnostic criteria and gestation, the prevalence of antenatally detected hydronephrosis (ANH) ranges from 0.6 to 5.4%.[1–6] The condition is bilateral in 17-54% and additional abnormalities are occasionally associated.[7–9] The outcome of ANH depends on the underlying etiology [Table 1].[10] Although ANH resolves by birth or during infancy in 41-88% patients[79–11] urological abnormalities requiring intervention are identified in 4.1-15.4%[6812] and rates of vesicoureteric reflux (VUR) and urinary tract infections (UTI) are several-fold higher.[713] It is important to distinguish infants with significant illness that require long-term follow-up or surgery, from those with transient hydronephrosis and minimum need for invasive investigations.

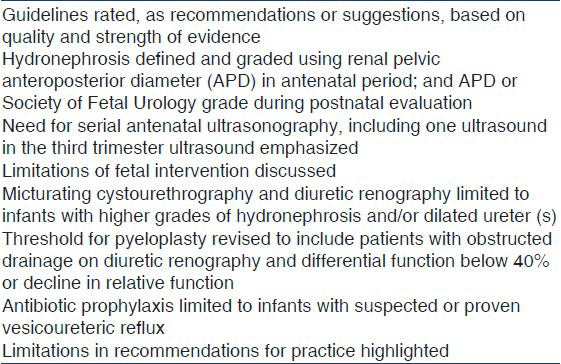
Guidelines from the Indian Society of Pediatric Nephrology (ISPN) on management of ANH were published in 2000.[14] During the last decade, there is better understanding regarding its often benign natural history and risk factors for postnatal pathology. Recommendations from other expert groups, including the Society for Fetal Urology (SFU) have been published.[1015] This document revises the ISPN guidelines and has been simultaneously published in the February 2013 issue of the Indian Pediatrics.
Materials and Methods
A literature search of PubMed, EMBASE and the Cochrane Library databases from 1990-2011 was performed for research articles on children with ANH. The findings were presented to an invited group of pediatric nephrologists, surgeons and radiologists, and an expert each from fetal medicine and nuclear medicine on 6 January 2012 in New Delhi. Based on the strength and consistency of evidence, the studies were rated from A to D as follows:
-
Systematic review, well designed randomized controlled trials (RCT) or diagnostic studies without significant limitations
-
RCT or diagnostic studies with methodological limitations; consistent evidence from observational studies
-
Small cohorts or case control studies; case series
-
Expert opinion; case reports
Subsequently, each guideline was assigned one of two levels of recommendation, based on assessment of relative benefit versus harm.
Level 1. Recommendation applicable to most subjects, based on consistent information confirming benefit over harm or vice versa.
Level 2. Suggestion or option based on equivocal or insufficient evidence and with unclear balance of benefit over harm, which may require modification when managing a patient.
The manuscript was circulated to participants of the meeting and to additional experts of the ISPN for approval. Important terms used in this document are described in Textbox 1.[1617] Table 2 lists salient differences between the present and previous recommendations.[14]
Antenatal Evaluation and Monitoring
Guideline 1: Diagnosis and grading of antenatal hydronephrosis
-
We recommend that ANH be diagnosed and its severity graded based on anteroposterior diameter (APD) of the fetal renal pelvis (1B).
-
ANH is present if the APD is ≥4 mm in second trimester and ≥7 mm in the third trimester.
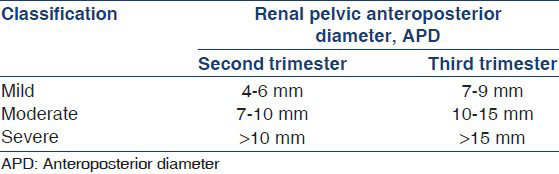
While the renal pelvic APD [Figure 1] varies with gestation, maternal hydration and bladder distension, it is an objective parameter with small intraobserver and interobserver variation.[18] ANH is present if fetal renal APD is ≥4 mm in the second trimester and ≥7 mm in third trimester.[10] Hydronephrosis is further graded as mild, moderate, or severe [Table 3]. A cross-sectional study shows that the upper limit for normal renal APD during late gestation is 7 mm.[19] An APD cutoff ≥7 mm at 18-weeks or later distinguishes fetuses with postnatal reflux or obstruction from those without significant pathology.[2021] Renal APD thresholds of 5 mm and 8-10 mm in the second and third trimester, respectively were 100% sensitive in predicting the need for postnatal surgery,[22] compared with a study where third trimester threshold of 10 mm missed 25% cases of pelviureteric junction obstruction and 50% cases of VUR.[1] Others also propose that fetal renal APD greater than 4-5 mm in the second trimester and 7 mm in third trimester is abnormal.[623–25] While lower cut-offs for defining hydronephrosis increase the sensitivity for detecting anomalies, it reduces specificity.
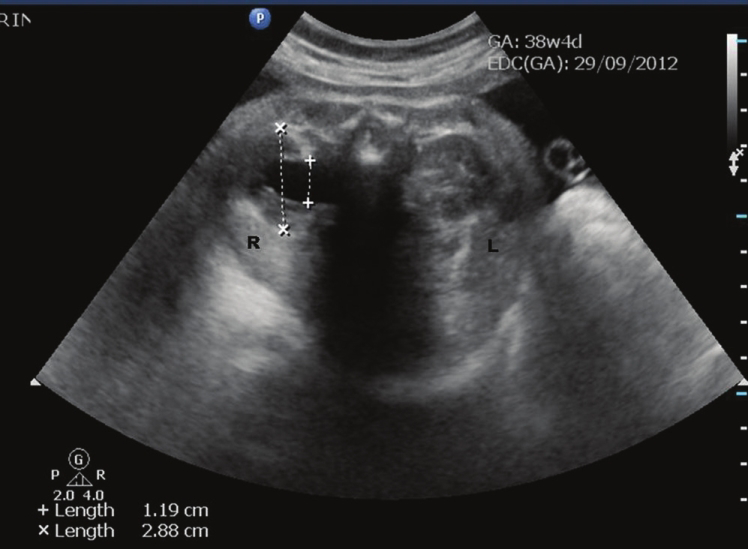
- Line diagram to measure fetal renal pelvic anteroposterior diameter. The APD is measured in the transverse axial image of the renal pelvis at level of the renal hilum. Antenatal ultrasound at 38-weeks showing right-sided hydronephrosis in transverse view (+---+): 11.9 mm. Anteroposterior diameter of the kidney (×---×): 28.8 mm
In a systematic analysis on 25 studies, Sidhu et al., showed that isolated ANH resolved or stabilized in 98% patients with APD < 12 mm as compared with 51% with larger APD.[26] In another meta-analysis on 1308 neonates from 17 studies, Lee et al., found that the risk of postnatal pathology increased with the degree of antenatal pelvic dilatation, from 11.9% for mild, 45.1% for moderate, and 88.3% for severe hydronephrosis.[7] The relationship was maintained for patients with pelviureteric junction obstruction or posterior urethral valves, but not for VUR and vesicoureteric junction obstruction. Other studies confirm that the severity of hydronephrosis correlates positively with postnatal hydronephrosis, need for surgery and risk of UTI, and negatively with spontaneous resolution.[5723252728] While fetuses with minimal pelvic dilatation (5-9 mm) have low risk of postnatal pathology,[2930] APD > 15 mm at any gestation represents severe hydronephrosis and requires close follow-up.[7822232531–33]
Guideline 2: Additional prenatal evaluation
-
If ANH is detected, we recommend that the ultrasound at 16-20 weeks gestation also include evaluation for lower urinary tract obstruction, renal dysplasia, and extrarenal structural malformations (1C).
-
We recommend that fetuses with ANH, and a major structural anomaly or additional soft sign(s) be referred to an obstetric unit with facilities for genetic counseling and prenatal testing (1C).
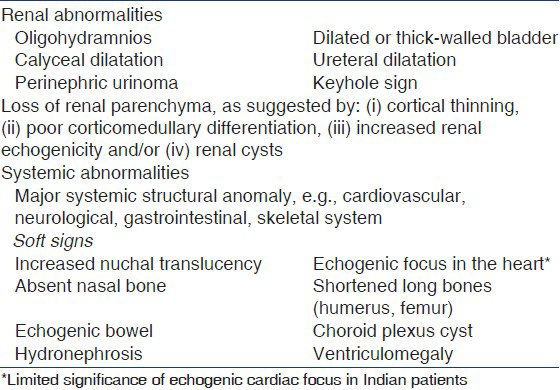
All fetuses with ANH should undergo detailed ultrasonography. The two signs that are useful in the diagnosis of lower urinary tract obstruction are oligohydramnios[34] and thick-walled or dilated bladder[35–41] [Table 3]. Other signs that predict postnatal pathology or need for surgery include bilateral hydroureteronephrosis, dilated posterior urethra, perinephric urinoma[4243] and progressive calyceal,[9234144] or ureteric dilatation.[94041] Features suggesting renal dysplasia and impaired renal function include abnormally large or small kidneys, oligohydramnios,[3645–47] parenchymal thinning,[48] cysts,[4950] and increased echogenicity.[34385152]
Antenatal ultrasonography, in conjunction with maternal age and first or second trimester blood screen, helps determine the risk of chromosomal disorders and need for karyotyping.[5354] The likelihood of aneuploidy in fetuses with isolated ANH is low[55–57] and karyotyping is not necessary. The risk of aneuploidy is increased in fetuses with ANH and a major structural anomaly[5859] or with one or more additional soft signs[1653] [Table 4]. Patients with these features require referral to a center with facilities for prenatal diagnosis and counseling. The decision regarding invasive testing is individualized, based on potential benefits and risks, and should occur at an appropriate time.
Guideline 3: Antenatal monitoring
-
In fetuses with unilateral hydronephrosis, we recommend that at least one follow-up ultrasound be performed in the third trimester (1B).
-
We suggest that fetuses with bilateral hydronephrosis be monitored frequently (2C). The frequency of monitoring varies from 4 to 6 weeks, depending on gestation at which ANH was detected, its severity and presence of oligohydramnios.
The gestation at which hydronephrosis is detected and its course on sequential ultrasound scans has prognostic value.[213260–62] Almost 80% of fetuses diagnosed in the second trimester show resolution or improvement of findings[62132] with low likelihood of postnatal sequelae.[432] Patients with persistence or worsening hydronephrosis in the third trimester show higher rates of postnatal pathology and require close follow-up.[45621233262] Sairam et al., found that 88% cases with mild ANH resolved in utero or neonatal period, while one in three neonates with moderate to severe hydronephrosis persisting in the third trimester required postnatal surgery.[6] Hence, an ultrasound in the third trimester is valuable for identifying fetuses that require postnatal evaluation and follow-up.
The risk of in utero worsening is higher for bilateral than for unilateral disease.[60] While a recent report suggests that patients with mild to moderate isolated bilateral hydronephrosis have a favorable outcome,[63] close follow-up is necessary since a proportion may show progression or require surgery.[40] Although there are limited studies that address frequency of monitoring,[7] we suggest that fetal imaging be repeated every 4-6 weeks depending on severity of hydronephrosis, gestation and presence of oligohydramnios. Fetuses with findings suggestive of lower urinary tract obstruction (bilateral hydroureteronephrosis, dilated bladder and oligohydramnios) might require even more frequent monitoring.
Guideline 4: Fetal intervention
-
We suggest that diagnostic and therapeutic interventions be considered for fetuses with suspected lower urinary tract obstruction and oligohydramnios only at specialized centers, following one-to-one counseling (2A).
-
Termination of pregnancy is not recommended in fetuses with unilateral or bilateral ANH, except in presence of extrarenal life threatening abnormality (1D).
If antenatal ultrasonography shows evidence of lower urinary tract obstruction (e.g., bilateral hydroureteronephrosis, dilated bladder, oligohydramnios), parents should be referred to specialized centers for counseling regarding prenatal diagnostic and therapeutic interventions. The predominant cause for lower urinary tract obstruction is posterior urethral valves in male fetuses. Fetal vesicocentesis, done on two or more occasions, allows estimation of urinary electrolytes, β2 microglobulin, and osmolality that predict renal maturity and function.[64–66] Decreasing levels of sodium (<100 mEq/l), calcium (<8 mg/dl), osmolarity (<200 mOsm/l), β2 microglobulin (<4 mg/l), and protein (<20 mg/dl) identify fetuses that are likely to benefit from therapeutic interventions.[67] In fetuses with suspected lower urinary obstruction and favorable indices, parents should be counseled regarding the role of vesicoamniotic shunting or in utero endoscopic ablation of valves.[106869]
The benefits of such intervention, usually performed during mid-second trimester, are equivocal. Meta-analyses show that prenatal bladder drainage, by vesicoamniotic shunt, improves perinatal survival in fetuses with severe obstruction, with benefits chiefly in those with poor predicted prognosis.[7071] There is no evidence that this intervention improves long term renal outcome or reduces mortality in fetuses with less severe disease.[687273] Moreover, vesicocentesis and other interventions carry considerable risk of fetal loss, chorioamnionitis, and preterm labor. While current evidence is insufficient, ongoing trials shall provide clarity on the efficacy and safety of these procedures.[74]
Pregnancy in fetuses with unilateral or bilateral ANH should proceed to term, except if complicated by severe oligohydramnios or major structural anomalies. Early delivery is not indicated, and carries risks of prematurity and low birth weight.
Postnatal Evaluation and Management
Guideline 5: Timing of initial ultrasound
-
We recommend that all newborns with history of ANH should have postnatal ultrasound examination within the first week of life (1B).
-
In neonates with suspected posterior urethral valves, oligohydramnios or severe bilateral hydronephrosis, ultrasonography should be performed within 24-48 h of birth (1C).
-
In all other cases, the ultrasound should be performed preferably within 3-7 days, or before hospital discharge (1C).
All newborns with a history of ANH, including those in whom it had resolved prenatally, should undergo postnatal evaluation.[1025] Reports suggest that hydronephrosis that has resolved postnatally does not merit prolonged follow-up and has satisfactory outcome.[7576] In a cohort of 130 infants with ANH and normal postnatal ultrasound, followed for 2 years without prophylaxis, the outcome was satisfactory without progression of hydronephrosis or occurrence of UTI.[76] Patients with persistent postnatal hydronephrosis require additional evaluation, the intensity of which is determined by the severity of findings.[15710] A systematic review of 31 studies concluded that the risk of postnatal pathology was 10.8% in infants with a normal postnatal ultrasound, compared to 54.7% in those with persisting hydronephrosis.[11] In another study, the negative predictive value of a normal postnatal ultrasound for UTI was 98.9%.[77] Nepple et al., showed that VUR was twice as likely to resolve in patients with normal postnatal ultrasound compared with those with abnormal findings.[78]
It is emphasized that an ultrasound in the first few days of life underestimates the degree of pelvic dilatation due to dehydration and a relatively low urine output.[7980] Despite this limitation, an early ultrasound, within 24-48 h of birth, is necessary in neonates with suspected lower urinary tract obstruction, oligohydramnios and bilateral severe hydronephrosis or severe hydronephrosis in a solitary kidney.[10] In others, the first ultrasound examination should ideally be delayed until the end of first week. Since there is a risk that a proportion of patients might be lost to follow-up,[81] we propose that neonates with unilateral or mild to moderate bilateral hydronephrosis be screened by ultrasonography prior to hospital discharge.
Guideline 6: Diagnosis and grading of postnatal hydronephrosis
-
We recommend that assessment of severity of postnatal hydronephrosis be based on the classification proposed by SFU or anteroposterior diameter of the renal pelvis (1B).
-
We suggest that ultrasonography should include evaluation for calyceal or ureteric dilation, cortical cysts and enhanced renal echogenicity, and bladder wall abnormalities (2D).
Common classifications for diagnosis and grading of postnatal hydronephrosis are those based on measurement of renal pelvic APD[78283] and that proposed by the SFU.[84] The latter assesses renal pelvic fullness, dilatation of major and minor calyces and cortical thickness [Figure 2]. Neonatal hydronephrosis is defined as SFU grade ≥ 1 or renal APD ≥ 7 mm. There are limited studies that have compared SFU and APD based classification systems. A systematic review concluded that 98% patients with SFU grade 1-2 or APD <12 mm resolved, compared with 51% with APD >12 mm or SFU 3-4.[26] A retrospective review showed correlation between APD <10 mm in late third trimester and SFU grade <2 on postnatal ultrasonography.[62]
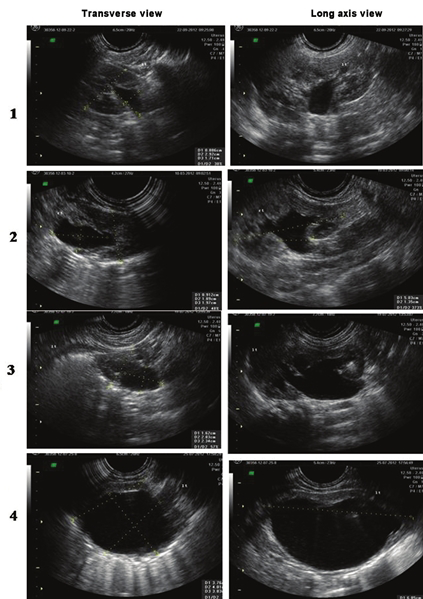
- Postnatal ultrasounds depicting the different grades of hydronephrosis according to the Society of Fetal Urology classification. Grade 1: Slight separation of the central renal echo complex. Grade 2: Renal pelvis is further dilated and a single or a few calyces may be visualized. Grade 3: Renal pelvis is dilated and there are fluid filled calyces throughout the kidney, but renal parenchyma is of normal thickness. Grade 4: As grade 3, but renal parenchyma over the calyces is thinned
Grading the severity of hydronephrosis enables identification of infants that require close follow-up. Multiple studies suggest that mild isolated unilateral or bilateral hydronephrosis with APD <9-11 mm is a frequent finding, which is unlikely to be associated with obstruction and has favorable prognosis.[7316385–89] In a systematic review, Paserotti et al., showed that the risk of postnatal pathology increased progressively from 29.6% with mild postnatal hydronephrosis to 96.3% in severe hydronephrosis.[11] Results of a meta-analysis showed that isolated ANH was five times more likely to stabilize if associated with SFU grade 1-2 or APD <12 mm than with SFU grade 3-4 or APD >12 mm.[26] In a retrospective review, Chertin found that SFU grade 3-4 were associated with high odds for surgery.[90]
Ultrasonography should include evaluation for calyceal or ureteric dilation, cortical cysts and echogenicity, bladder wall abnormalities, ureterocele and bladder emptying.[9192] The presence of calyceal and/or ureteric dilatation has high (87-96%) specificity but low sensitivity (37-54%) for detecting grade III-V VUR.[93] Increased parenchymal echogenicity, loss of corticomedullary differentiation and presence of cortical cysts on postnatal ultrasound predict impaired renal function or dysplasia in patients with pelviureteric obstruction and posterior urethral valves.[94–97]
Guideline 7: Postnatal monitoring
-
We recommend that neonates with normal ultrasound examination in the first week of life should undergo a repeat study at 4-6 weeks (1C).
-
We recommend that infants with isolated mild unilateral or bilateral hydronephrosis (APD < 10 mm or SFU grade 1-2) should be followed by sequential ultrasound alone, for resolution or progression of findings (1C).
A single ultrasound in the first week of life might not detect all abnormalities of the kidneys or urinary tract, due to low urine flow secondary to dehydration and low glomerular filtration rate (GFR). An ultrasound at 6 weeks is more sensitive and specific for obstruction, than that in the first week of life.[88] All newborns with a normal ultrasound at first week, therefore, require a repeat study at 4-6 weeks.[10] The presence of two normal postnatal renal ultrasounds excludes presence of significant renal disease including dilating VUR.[2198]
The frequency of subsequent monitoring in patients with persistent postnatal hydronephrosis depends on its severity, and includes evaluation for increasing pelvicalyceal or ureteric dilatation and cortical thinning. A repeat ultrasound may show late worsening or recurrence of hydronephrosis in 1-5% patients.[99100] Since progression might occur in the first 2-years of life, and occasionally until 5-6 years,[101] follow-up studies are scheduled at 3-6 months, and then 6-12 monthly until resolution.[102]
Most patients with mild hydronephrosis (SFU grade 1-2; renal APD < 10 mm) do not have significant obstruction and maintain kidney function on the long-term. The intensity of evaluation for milder grades of hydronephrosis has therefore declined.[103–105] Recent cohorts with unilateral or bilateral hydronephrosis with APD up to 10 mm[106] or 15 mm[63107] have been followed successfully, relying on clinical features, ultrasonography and counseling parents for surveillance for UTI. Hydronephrosis resolves in most such patients during the first 2-years of life, and radiologic investigations or antibiotic prophylaxis is usually not necessary. The policy to follow these neonates with sequential ultrasonography to monitor for resolution of hydronephrosis therefore seems satisfactory.
Various experts propose that infants with renal APD exceeding 10 mm or SFU grade 3-4 at onset require close follow-up.[11016108109] Evaluation of the upper and/or lower urinary tract is limited to these patients and those showing increasing dilatation of renal pelvis, calyces or ureter, or thinning of cortical parenchyma.[9193–96]
Guideline 8: Micturating cystourethrogram
-
We recommend that a micturating cystourethrogram (MCU) be performed in patients with unilateral or bilateral hydronephrosis with renal pelvic APD > 10 mm, SFU grade 3-4 or ureteric dilatation (1B).
-
We recommend that MCU be performed early, within 24-72 h of life, in patients with suspected lower urinary tract obstruction (1D). In other cases, the procedure should be done at 4-6 weeks of age.
-
We recommend MCU for infants with antenatally detected hydronephrosis who develop a urinary tract infection (1C).
Lower urinary tract obstruction (most commonly posterior urethral valves in boys; occasionally bilateral ureteroceles) is an important cause of ANH and requires prompt management. Ultrasonographic findings of posterior urethral valves are: (i) bilateral hydroureteronephrosis, (ii) dilated, thick-walled bladder that fails to empty, and (iii) dilated posterior urethra. Since these patients are at risk for progressive kidney disease and recurrent UTI, an early MCU (within 1-3 days of life) enables prompts intervention.
VUR is present in 8-38% patients with unilateral or bilateral ANH, as compared with < 1% in the general population.[7110111] While there is increased risk of UTI, there is lack of evidence that antibiotic prophylaxis in patients with mild VUR confers clinical benefit.[112] Multiple studies and a systematic review suggest that the severity of ANH does not correlate with the grade of reflux,[725113] and that patients with VUR may have normal postnatal ultrasound.[112393114] However, renal pelvic APD exceeding 10-11 mm is useful in identifying patients with severe VUR.[98115116]
We recommend that MCU be restricted to infants with moderate to severe hydronephrosis (SFU grade 3-4, or renal APD >10 mm), dilated ureter(s), or bladder or urethral abnormalities. Although evidence for timing is lacking, the procedure is performed at 4-6 weeks of age, unless lower urinary tract obstruction is suspected (see above). MCU is also required in patients with history of milder grades of ANH who show worsening hydronephrosis, progressive parenchymal thinning or occurrence of UTI.[117] Physicians should be aware that this investigation is associated with risks of UTI[118] and exposure to radiation.[119]
Guideline 9: Diuretic renography
-
We recommend that infants with moderate to severe unilateral or bilateral hydronephrosis (SFU grade 3-4, APD >10 mm) who do not show VUR should undergo diuretic renography (1C).
-
We suggest that infants with hydronephrosis and dilated ureter(s) and no evidence of VUR undergo diuretic renography (2C).
-
The preferred radiopharmaceuticals are 99mTc-mercaptoacetyltriglycine (99mTc-MAG3), 99mTc-ethylenedicysteine (99mTc-EC) or 99mTc-diethylenetriaminepentaacetic acid (DTPA) (2D). The differential function is estimated and renogram curve inspected for pattern of drainage.
-
We suggest that diuretic renography be performed after 6-8 weeks of age (2D). The procedure may be repeated after 3-6 months in infants where ultrasound shows worsening of pelvicalyceal dilatation (2D).
Pelviureteric junction obstruction should be considered in infants with hydronephrosis, where dilating VUR is excluded. The likelihood of detecting obstruction is considerably higher in patients with SFU grade 4 or renal APD exceeding 20-30 mm.[72526] The possibility of vesicoureteric junction obstruction or megaureter is considered in patients with hydronephrosis and dilated ureter where MCU is normal. Patients with VUR and worsening hydronephrosis also require evaluation for pelviureteric junction obstruction, since the two may coexist in 7-18% patients.[120]
Diuretic renography allows differentiation between obstructive and non-obstructive hydronephrosis and estimating relative renal function.[121] Radiopharmaceuticals such as 99mtechnetium mercaptoacetyltriglycine (99mTc-MAG3) or ethylenedicysteine (99mTc-EC) are preferred, since they show greater renal extraction and higher kidney to background ratio compared to diethylenetriaminepentaacetic acid (99mTc-DTPA).[122–124] However, DTPA is inexpensive and widely available.
Since immaturity of renal function results in reduced radiotracer uptake, renography is done at 6-8 weeks of life but may be performed earlier in patients with severe hydronephrosis and cortical thinning. Textbox 2 lists guidelines for renography.[121125] Intravenous hydration and bladder catheterization are not necessary,[121126127] the latter indicated in patients with poor bladder emptying on late films (neurogenic bladder), severe bilateral reflux or megaureters. A normal renogram curve is characterized by an early peak (2-5 min), rapidly descending phase and almost complete renal emptying by 20 min. Drainage is influenced by state of hydration, and composite and differential kidney function.[121127] The presence of satisfactory drainage spontaneously, or following IV frusemide and micturition excludes significant obstruction. An obstructive pattern is defined by an ascending or plateau phase over 20 min, that fails to empty following diuretic administration and on post-micturition views.[121127] Differential renal function is estimated; values between 45% and 55% are considered normal.[128129] An initial differential function below 35-40% in the kidney with obstructed drainage signifies impaired function.[130] Other features that suggest obstruction include ipsilateral supranormal differential renal function (≥55%)[131132] and prolonged time to clear 50% of the radionuclide (t1/2 > 20 min).[133]
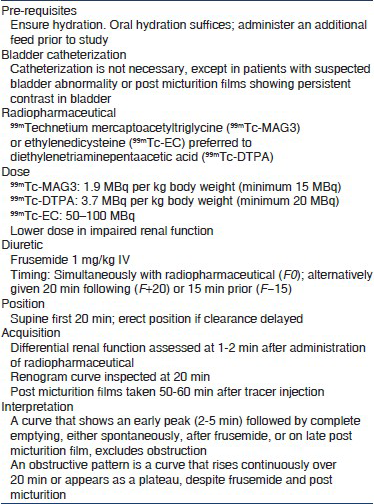
Many patients require repeat renography, when change in differential function and drainage pattern is compared.[130] The timing of the repeat procedure is not defined, and varies with patient age, initial renal function and persistence or worsening of ultrasonographic findings. The tracer used for the first renogram and timing of diuretic administration should be similar during serial evaluations.
Guideline 10: Indications for surgery
-
We recommend that infants with lower urinary tract obstruction be immediately referred to a surgeon for appropriate intervention (1C).
-
We suggest that surgery be considered in patients with obstructed hydronephrosis, and either reduced differential renal function or its worsening on repeat evaluation (2C).
-
We suggest that surgery be considered in patients with bilateral hydronephrosis or hydronephrosis in solitary kidney showing worsening dilatation and deterioration of function (2D).
Infants with posterior urethral valves require early urethral catheterization, correction of electrolyte abnormalities, treatment for possible complications and referral for surgical intervention.[134] Cystoscopic ablation of the urethral valves is recommended.[135136]
While most experts suggest that pyeloplasty be considered in patients showing obstructed drainage and differential function below 40%,[137–139] others propose surgery at differential function below 35%,[140] or an obstructed renogram with prolonged t1/2 > 20 min.[141]
Conservative management is appropriate for infants with an obstructive pattern on diuretic renography and differential function exceeding 40%.[90] Serial ultrasonography is recommended[10142] and repeat renography done if there is persistent or progressive hydronephrosis or parenchymal thinning.[143144] A reduction of differential renal function by more than 5-10% correlates with declining renal function, and the need for pyeloplasty.[130145] Other indications for surgery include presence of pain, palpable renal lump or recurrent febrile UTI.[117] The presence of large APD exceeding 20-30 mm predicts the need for surgery in 50-55% patients.[139146–148] Surgery allows preservation of renal function in the majority; predictors of unsatisfactory outcome include baseline differential function <30%[149] and renal APD >50 mm with dilated calyces.[148]
Few reports describe the management of neonates with bilateral severe hydronephrosis secondary to pelviureteric junction obstruction. While in unilateral hydronephrosis, the affected kidney is compared with normal, in bilateral hydronephrosis the function of both kidneys is potentially at risk. Careful follow-up with serial ultrasonography and radionuclide studies for worsening hydronephrosis and declining differential function and estimated GFR is recommended. While case series underscore the need for early intervention,[150] patients with mild to moderate hydronephrosis have successfully been managed conservatively.[151] Most surgeons prefer to operate first on the kidney that is more severely affected.[152153]
Guideline 11: Antibiotic prophylaxis
-
We recommend that parents of all infants with antenatal or postnatal hydronephrosis be counseled regarding the risk of urinary tract infections and need for prompt management (1B).
-
We recommend that infants with postnatally confirmed moderate or severe hydronephrosis (SFU 3-4; renal APD > 10 mm) or dilated ureter receive antibiotic prophylaxis while awaiting evaluation (1C).
-
We recommend that all patients detected to have VUR receive antibiotic prophylaxis through the first year of life (1B).
Infants with ANH, including where hydronephrosis has resolved postnatally, have an increased risk of UTI.[7154] Walsh et al., retrospectively estimated that the relative risk of developing pyelonephritis in these infants was 11.8 (95% confidence interval 6.8-20.5).[13] The rates of UTI have varied, based on severity of hydronephrosis, duration of follow-up and antibiotic use. Infections were reported in 1.6-7.2% infants with ANH administered antibiotic prophylaxis[889154] and 3.9-10% of those not receiving prophylaxis.[376398154] In patients with isolated hydronephrosis (postnatal renal pelvic APD 5-15 mm) followed without prophylaxis, the frequency of UTI was similar in patients with bilateral (9%) or unilateral (10%) disease.[63] Parents should be counseled regarding the increased risk of UTI, and the need for prompt diagnosis and treatment.
Coelho et al., reported that infants with postnatal renal pelvic APD of 10 mm or more have significantly increased risk of infections (relative risk 2.6, 95% confidence interval 1.2-5.8) compared with those with mild hydronephrosis.[106] Other studies have confirmed this finding[851155] and suggest that most UTI occur within the first 6 months of life.[156] While a significant proportion of infections occur in the context of underlying VUR, other risk factors include ureteric dilatation[155156] and underlying obstruction.[155157] In a recent meta-analysis including 3876 infants, it was demonstrated that neonates with high grade hydronephrosis receiving antibiotic prophylaxis have a significantly lower rate of UTI when compared to untreated neonates (14.6% versus 28.9%; P <0.01), while the rates of UTI were low for neonates with low grade hydronephrosis, regardless of status of antibiotic prophylaxis (2.2% on prophylaxis versus 2.8% without).[158] Thus, only 7 patients with high-grade hydronephrosis are required to be treated with antibiotic prophylaxis in order to prevent one UTI.
Patients with moderate or severe hydronephrosis and/or dilated ureter should receive antibiotic prophylaxis while awaiting investigations. Since the risk of UTI is low with mild hydronephrosis, antibiotic prophylaxis is not necessary in these infants.[863]
The efficacy of antibiotic prophylaxis in preventing UTI in patients with VUR has been questioned.[159–161] While awaiting results of further studies, the ISPN currently recommends that infants with VUR should receive antibiotic prophylaxis, the duration determined by the grade of reflux and occurrence of breakthrough infections.[162] The American Urological Association also recommends that antibiotic prophylaxis be given to infants with VUR grade III-V that is identified through screening.[104] They further suggest that, although evidence is limited, infants with lower grades of VUR (grade I-II) may also receive prophylaxis. In view of difficulties of detecting UTI in infancy and risks of renal scarring,[163] we recommend antibiotic prophylaxis for all infants with VUR detected through screening. Antibiotics that are preferred include cephalexin (10 mg/kg/d) during the first 3 months of life, and cotrimoxazole (1-2 mg/kg/d) or nitrofurantoin (1 mg/kg/d) later.
In absence of prospective controlled studies, there is variability in practice regarding use of antibiotics in children with moderate to severe obstructive hydronephrosis.[164] The rates of UTI were 0-4.3% in studies on patients with severe hydronephrosis due to pelviureteric junction obstruction or megaureter, managed without prophylaxis.[165166] Madden et al., showed that the rates of UTI were similar at 14% and 16% in infants followed with or without prophylaxis, respectively.[138] Other studies show that 19-36.2% of patients with moderate or severe obstructive hydronephrosis have UTI.[157158] Further studies are necessary to determine the benefit of antibiotic prophylaxis in patients with obstructive hydronephrosis.
Risk of radiation exposure
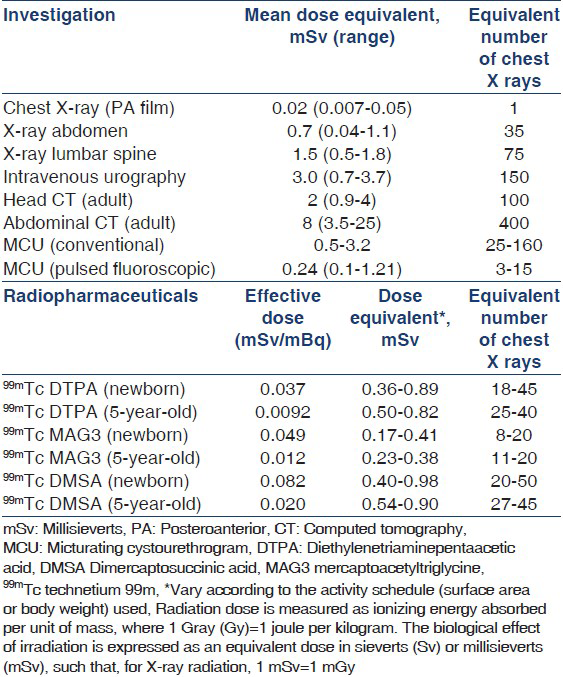
Radiocontrast and radionuclide studies are associated with considerable risk of radiation exposure. The exposure following these studies is several-fold higher than a chest radiograph.[167–169] Recent findings from a large cohort of patients undergoing repeated CT scans show that cumulative doses of 50-60 mGy (equivalent to 50-60 mSv of X-ray radiation) were associated with 3-fold increased risk of leukemia and brain cancer.[170] Physicians should be aware of the risks associated with these investigations [Table 5].[121169171172] Repeat radionuclide and radiocontrast studies should be done only if these are likely to provide clinically relevant information that cannot be obtained by ultrasonography. Intravenous urography should not be used as an alternative to radionuclide scans. Magnetic resonance urography provides useful information, but is not freely available, requires sedation, and is associated with risks in patients with impaired renal function.
Conclusions
Figures 3 and 4 summarize the guidelines for management of patients with ANH. While a significant proportion have transient hydronephrosis that resolves in utero or postnatally, neonates with persistent hydronephrosis require follow-up. All neonates with hydronephrosis should undergo urinalysis, measurement of blood pressure, and estimation of serum creatinine. Infants with moderate to severe hydronephrosis are screened for urinary tract obstruction or VUR. The initial evaluation aims to detect patients with bladder obstruction, which requires prompt intervention. Decisions regarding surgical intervention, in other patients with obstructive hydronephrosis, depend on a combination of clinical and laboratory features, and results of sequential ultrasonography and diuretic renography.
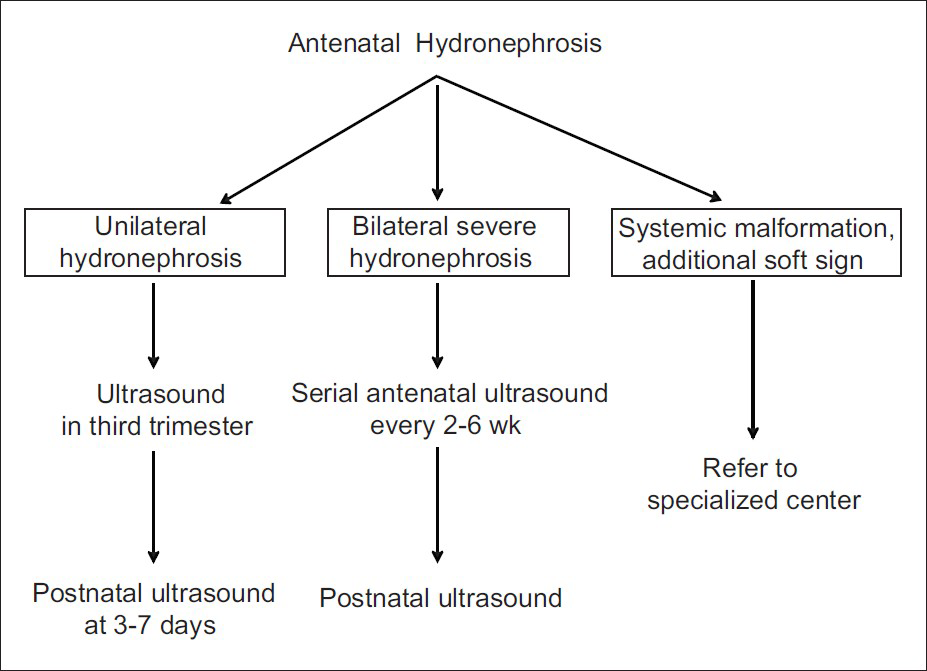
- Prenatal monitoring in patients with antenatally detected hydronephrosis. All fetuses with ANH should undergo at least one ultrasound in third trimester, and its severity is graded according to renal pelvic anteroposterior diameter [Table 3]. Fetuses with bilateral hydronephrosis need monitoring through pregnancy, the frequency of which depends on severity of findings and presence of oligohydramnios. Those with oligohydramnios or other systemic abnormalities should be referred to specialized centers. While all newborns with antenatally detected hydronephrosis should undergo ultrasonography in the first week of life, those with suspected bladder obstruction should undergo postnatal ultrasonography within 48 hr of birth
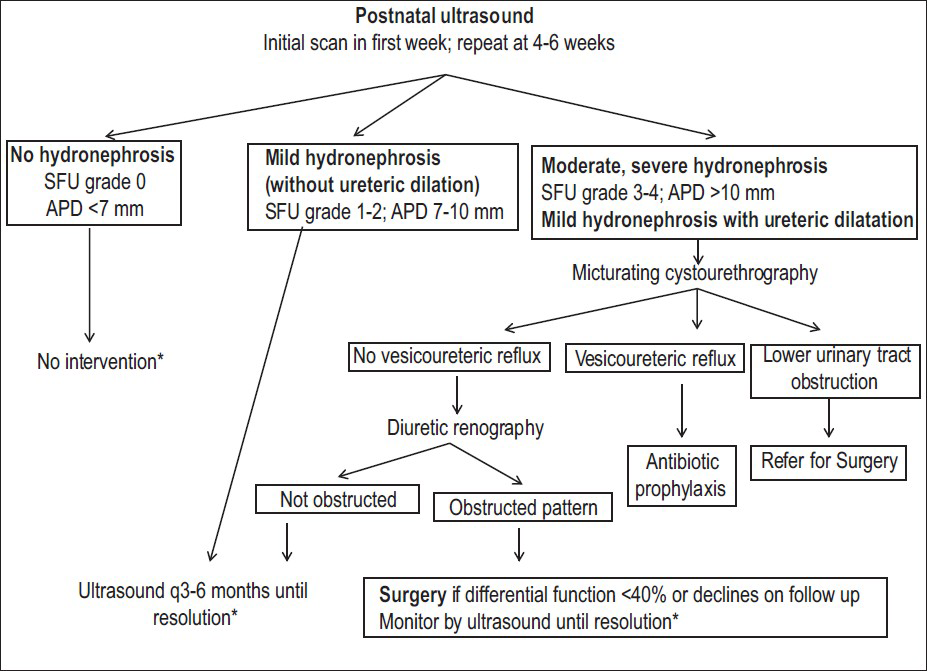
- Postnatal evaluation in patients with antenatal hydronephrosis. A postnatal ultrasound is recommended at 3-7 days except in suspected lower urinary tract obstruction, where it is done earlier. Postnatal hydronephrosis is classified using Society of Fetal Urology grade or renal pelvic anteroposterior diameter (APD). Infants with normal findings should undergo a repeat study at 4-6 weeks. Patients with isolated mild hydronephrosis (unilateral or bilateral) should be followed with sequential ultrasounds, at 3- and 6-months, followed by 6-12 monthly until resolution; those with worsening hydronephrosis require closer evaluation. Patients with higher grades of hydronephrosis or dilated ureter (s) are screened for underlying obstruction or VUR. Diuretic renography is useful in detecting pelviureteric junction or vesicoureteric junction obstruction and determining the need for surgery. *Parents of infants with hydronephrosis should be counseled regarding the risk of urinary tract infections
Writing Committee
Aditi Sinha, Department of Pediatrics, All India Institute of Medical Sciences (AIIMS), New Delhi; Arvind Bagga, Department of Pediatrics, AIIMS, New Delhi; Anurag Krishna, Max Institute of Pediatrics and Pediatric Surgery, New Delhi; Minu Bajpai, Department of Pediatric Surgery, AIIMS, New Delhi; M Srinivas, Department of Pediatric Surgery, AIIMS, New Delhi; Rajesh Uppal, Uppal Radiology Center, New Delhi; Indira Agarwal, Department of Pediatrics, Christian Medical College, Vellore
Expert Group
Kamran Afzal, Aligarh
Indira Agarwal, Vellore
Vinay Agarwal, New Delhi
Uma S. Ali, Mumbai
Kanav Anand, New Delhi
Sanjeev Bagai, New Delhi
Arvind Bagga, New Delhi, Convener
Meenu Bajpai, New Delhi
Sushmita Banerjee, Kolkata
Tathagata Bose, Ahmedabad
Deepika Deka, New Delhi
Arpana Iyengar, Bangalore
Ashima Gulati, New Delhi
Sanjeev Gulati, New Delhi
Arun Kumar Gupta, New Delhi
Pankaj Hari, New Delhi
Anurag Krishna, New Delhi
Manish Kumar, New Delhi
Rakesh Kumar, New Delhi
Madhuri Kanitkar, New Delhi
Mukta Mantan, New Delhi
Amarjeet Mehta, Jaipur
BR Nammalwar, Chennai
Saroj K Patnaik, Bangalore
PK Pruthi, New Delhi
Kishore Phadke, Bangalore
B Rath, New Delhi
Abhijeet Saha, New Delhi
Ashu Bhalla Seith, New Delhi
Sidharth Sethi, New Delhi
Jyoti Sharma, Pune
Aditi Sinha, New Delhi
Rajiv Sinha, Kolkata
Shalini Sinha, New Delhi
M. Srinivas, New Delhi
Rajendra Nath Srivastava, New Delhi
Rajesh Uppal, New Delhi
Susan Uthup, Thiruvananthapuram
Anand S. Vasudev, New Delhi
Anil Vasudevan, Bangalore
M Vijayakumar, Chennai
Amarjeet Mehta, Jaipur
Acknowledgment
We thank Dr. Paul Winyard, Head of Nephro-Urology Unit, UCL Institute of Child Health, London (UK) for reviewing the manuscript.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Antenatally detected urinary tract abnormalities: More detection but less action. Pediatr Nephrol. 2008;23:897-904.
- [Google Scholar]
- Clinical relevance and implications of antenatal hydronephrosis. Arch Dis Child Fetal Neonatal Ed. 1997;76:F31-4.
- [Google Scholar]
- A study on fetal urinary tract anomaly: Antenatal ultrasonographic diagnosis and postnatal follow-up. J Obstet Gynaecol Res. 1996;22:569-73.
- [Google Scholar]
- Fetal hydronephrosis; prevalence, natural history and postnatal consequences in an unselected population. Acta Obstet Gynecol Scand. 2007;86:1463-6.
- [Google Scholar]
- Antenatal ultrasonography to detect fetal renal abnormalities: A prospective screening programme. BMJ. 1989;298:1421-3.
- [Google Scholar]
- Natural history of fetal hydronephrosis diagnosed on mid-trimester ultrasound. Ultrasound Obstet Gynecol. 2001;17:191-6.
- [Google Scholar]
- Antenatal hydronephrosis as a predictor of postnatal outcome: A meta-analysis. Pediatrics. 2006;118:586-93.
- [Google Scholar]
- The long-term outcome of antenatal hydronephrosis up to 15 millimetres justifies a noninvasive postnatal follow-up. Acta Paediatr. 2008;97:708-13.
- [Google Scholar]
- The 4 year outcome following the demonstration of bilateral renal pelvic dilatation on pre-natal renal ultrasound. Br J Radiol. 1999;72:265-70.
- [Google Scholar]
- The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol. 2010;6:212-31.
- [Google Scholar]
- The predictive value of the first postnatal ultrasound in children with antenatal hydronephrosis. J Pediatr Urol. 2011;7:128-36.
- [Google Scholar]
- Prognostic significance of antenatally detected fetal pyelectasis. Ultrasound Obstet Gynecol. 1996;7:424-8.
- [Google Scholar]
- Antenatal hydronephrosis and the risk of pyelonephritis hospitalization during the first year of life. Urology. 2007;69:970-4.
- [Google Scholar]
- Indian Pediatric Nephrology Group, Indian Academy of Pediatrics. Consensus statement on management of antenatally detected hydronephrosis. Indian Pediatr. 2001;38:1244-51.
- [Google Scholar]
- Investigation and management of antenatally detected hydronephrosis. Can Urol Assoc J. 2009;3:69-72.
- [Google Scholar]
- Diagnostic Imaging Committee, Society of Obstetricians and Gynaecologists of Canada, Genetics Committee, Society of Obstetricians and Gynaecologists of Canada. Fetal soft markers in obstetric ultrasound. J Obstet Gynaecol Can. 2005;27:592-636.
- [Google Scholar]
- The second trimester genetic sonogram. Am J Med Genet C Semin Med Genet. 2007;145C:62-72.
- [Google Scholar]
- Antenatal ultrasonographic anteroposterior renal pelvis diameter measurement: Is it a reliable way of defining fetal hydronephrosis? Obstet Gynecol Int. 2011;2011:861-5.
- [Google Scholar]
- Charts of fetal size: Kidney and renal pelvis measurements. Prenat Diagn. 2003;23:891-7.
- [Google Scholar]
- Results of systematic screening for minor degrees of fetal renal pelvis dilatation in an unselected population. Am J Obstet Gynecol. 2003;188:242-6.
- [Google Scholar]
- Diagnostic value of anteroposterior diameter of fetal renal pelvis during second and third trimesters in predicting postnatal surgery among Korean population: Useful information for antenatal counseling. Urology. 2012;79:1132-7.
- [Google Scholar]
- Congenital hydronephrosis: Correlation of fetal ultrasonographic findings with infant outcome. Am J Obstet Gynecol. 1991;165:384-8.
- [Google Scholar]
- Postnatal management of infants with antenatally detected hydronephrosis. Pediatr Nephrol. 2005;20:1253-9.
- [Google Scholar]
- Outcome of isolated antenatal hydronephrosis: A systematic review and meta-analysis. Pediatr Nephrol. 2006;21:218-24.
- [Google Scholar]
- The yield of early postnatal ultrasound scan in neonates with documented antenatal hydronephrosis. Am J Perinatol. 2011;28:613-8.
- [Google Scholar]
- Outcome of fetal renal pelvic dilatation diagnosed during the third trimester. Ultrasound Obstet Gynecol. 2005;25:483-8.
- [Google Scholar]
- Minimal hydronephrosis in the fetus: Clinical significance and implications for management. J Urol. 1996;155:2047-9.
- [Google Scholar]
- Smith SE Mild dilatation of the fetal kidney: A follow-up study. Br J Urol. 1994;74:236-9.
- [Google Scholar]
- The magnitude of fetal renal pelvic dilatation can identify obstructive postnatal hydronephrosis, and direct postnatal evaluation and management. J Urol. 2006;176:724-7.
- [Google Scholar]
- Evaluation and follow-up of fetal hydronephrosis. J Ultrasound Med. 2001;20:1065-9.
- [Google Scholar]
- Prediction of the outcome of antenatally diagnosed hydronephrosis: A multivariable analysis. J Pediatr Urol. 2012;8:135-9.
- [Google Scholar]
- Increased renal echogenicity: A sonographic sign for differentiating between obstructive and nonobstructive etiologies of in utero bladder distension. J Urol. 1997;158:1026-9.
- [Google Scholar]
- Posterior urethral valves: Prenatal diagnostic signs and outcome. Urol Int. 2004;73:296-301.
- [Google Scholar]
- Predictive factors of fetal urethral obstruction: A multivariate analysis. Fetal Diagn Ther. 2000;15:180-6.
- [Google Scholar]
- Correlation between ultrasound and anatomical findings in fetuses with lower urinary tract obstruction in the first half of pregnancy. Ultrasound Obstet Gynecol. 2005;25:478-82.
- [Google Scholar]
- Keyhole sign: How specific is it for the diagnosis of posterior urethral valves? Ultrasound Obstet Gynecol. 2009;34:419-23.
- [Google Scholar]
- Postnatal outcome of fetal hydronephrosis: Implications for prenatal counselling. Indian J Urol. 2010;26:60-2.
- [Google Scholar]
- Late second trimester assessment of pyelectasis (SERP) to predict pediatric urological outcome is improved by checking additional features. J Matern Fetal Neonatal Med. 2006;19:295-303.
- [Google Scholar]
- Fetal urinoma: A case report and review of its clinical significance. J Ultrasound Med. 1994;13:989-91.
- [Google Scholar]
- How does the presence of antenatally detected caliectasis predict the risk of postnatal surgical intervention? Urology. 2012;80:203-6.
- [Google Scholar]
- Prognostic factors in fetal hydronephrosis: A multivariate analysis. Pediatr Nephrol. 1999;13:859-64.
- [Google Scholar]
- Prognostic factors in prenatally-detected posterior urethral valves: A multivariate analysis. Pediatr Surg Int. 2002;18:662-7.
- [Google Scholar]
- Amniotic fluid index and fetal bladder outlet obstruction.Do we really need more? J Urol. 2005;174:1657-60.
- [Google Scholar]
- Hydronephrosis with diffuse or segmental cortical thinning: Impact on renal function. J Urol. 2001;165:2293-5.
- [Google Scholar]
- Development of human fetal kidney in obstructive uropathy: Correlations with ultrasonography and urine biochemistry. Kidney Int. 1997;52:21-32.
- [Google Scholar]
- Fetal hyperechogenic kidney with normal amniotic fluid volume: A diagnostic dilemma. Prenat Diagn. 2005;25:553-8.
- [Google Scholar]
- Increased renal parenchymal echogenicity in the fetus: Importance and clinical outcome. Radiology. 1991;181:135-9.
- [Google Scholar]
- The genetic sonogram: A method of risk assessment for Down syndrome in the second trimester. J Ultrasound Med. 2002;21:1087-96.
- [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No.88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007;110:1459-67.
- [Google Scholar]
- The association between fetal pyelectasis on second trimester ultrasound scan and aneuploidy among 25,586 low risk unselected women. Prenat Diagn. 2002;22:1201-6.
- [Google Scholar]
- Isolated fetal pyelectasis and chromosomal abnormalities. Am J Obstet Gynecol. 2005;193:732-8.
- [Google Scholar]
- Second-trimester ultrasound to detect fetuses with Down syndrome: A meta-analysis. JAMA. 2001;285:1044-55.
- [Google Scholar]
- Prenatal diagnosis of chromosome abnormalities in the presence of fetal structural defects. Am J Med Genet. 1988;29:289-91.
- [Google Scholar]
- Should determination of the karyotype be systematic for all malformations detected by obstetrical ultrasound? Prenat Diagn. 2005;25:567-73.
- [Google Scholar]
- In utero progression of isolated renal pelvis dilation. Am J Perinatol. 1997;14:423-6.
- [Google Scholar]
- Prenatal diagnosis and management of mild fetal pyelectasis: Implications for neonatal outcome and follow-up. Eur J Obstet Gynecol Reprod Biol. 2005;118:154-9.
- [Google Scholar]
- Antenatal hydronephrosis: Thresholds of renal pelvic diameter to predict insignificant postnatal pelviectasis. Tech Urol. 1998;4:198-201.
- [Google Scholar]
- Natural history of bilateral mild isolated antenatal hydronephrosis conservatively managed. Pediatr Nephrol. 2012;27:1119-23.
- [Google Scholar]
- The nonpredictive value of fetal urinary electrolytes: Preliminary report of outcomes and correlations with pathologic diagnosis. Am J Obstet Gynecol. 1987;157:694-8.
- [Google Scholar]
- Fetal intervention in obstructive uropathy: Prognostic indicators and efficacy of intervention. Am J Obstet Gynecol. 1990;162:1239-44.
- [Google Scholar]
- Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: A systematic review. Ultrasound Obstet Gynecol. 2011;37:629-37.
- [Google Scholar]
- The current approach to the assessment of fetal renal function: Fact or fiction? Pediatr Nephrol. 1996;10:230-5.
- [Google Scholar]
- Fetal surgery for posterior urethral valves: Long-term postnatal outcomes. Pediatrics. 2001;108:E7.
- [Google Scholar]
- Fetal cystoscopy for severe lower urinary tract obstruction-Initial experience of a single center. Prenat Diagn. 2010;30:30-9.
- [Google Scholar]
- Prenatal bladder drainage in the management of fetal lower urinary tract obstruction: A systematic review and meta-analysis. Obstet Gynecol. 2003;102:367-82.
- [Google Scholar]
- Systematic review of the effectiveness of antenatal intervention for the treatment of congenital lower urinary tract obstruction. BJOG. 2010;117:382-90.
- [Google Scholar]
- Outcome analysis of vesicoamniotic shunting in a comprehensive population. J Urol. 2001;166:1036-40.
- [Google Scholar]
- Long-term outcomes in children treated by prenatal vesicoamniotic shunting for lower urinary tract obstruction. Obstet Gynecol. 2005;106:503-8.
- [Google Scholar]
- PLUTO trial protocol: Percutaneous shunting for lower urinary tract obstruction randomised controlled trial. BJOG. 2007;114:904-5.
- [Google Scholar]
- Postnatal investigation and outcome of isolated fetal renal pelvis dilatation. Arch Pediatr. 2009;16:1103-10.
- [Google Scholar]
- Brussels Free University Perinatal Nephrology Study Group. Long-term clinical outcome of infants with mild and moderate fetal pyelectasis: Validation of neonatal ultrasound as a screening tool to detect significant nephrouropathies. J Pediatr. 2004;144:759-65.
- [Google Scholar]
- Antenatal hydronephrosis: Negative predictive value of normal postnatal ultrasound-A 5-year study. Clin Radiol. 2003;58:964-70.
- [Google Scholar]
- The prognostic impact of an abnormal initial renal ultrasound on early reflux resolution. J Pediatr Urol. 2011;7:462-6.
- [Google Scholar]
- The fate of infant kidneys with fetal hydronephrosis but initially normal postnatal sonography. J Urol. 1989;142:661-2.
- [Google Scholar]
- Optimal timing of initial postnatal ultrasonography in newborns with prenatal hydronephrosis. J Urol. 2002;168:1826-9.
- [Google Scholar]
- Postnatal follow-up of antenatal hydronephrosis: A health-care challenge. J Perinatol. 2009;29:382-7.
- [Google Scholar]
- Urinary tract dilatation in utero: Classification and clinical applications. Radiology. 1986;160:645-7.
- [Google Scholar]
- Ultrasound grading of hydronephrosis: Introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993;23:478-80.
- [Google Scholar]
- Management in children of mild postnatal renal dilatation but without vesicoureteral reflux. Pediatr Nephrol. 2010;25:477-83.
- [Google Scholar]
- Renal pyelectasis in fetuses and neonates: Diagnostic value of renal pelvis diameter in pre- and postnatal sonographic screening. AJR Am J Roentgenol. 1997;168:1017-9.
- [Google Scholar]
- Diagnosis of obstructive hydronephrosis in infants: Comparison sonograms performed 6 days and 6 weeks after birth. AJR Am J Roentgenol. 1995;164:963-7.
- [Google Scholar]
- Mild antenatal hydronephrosis: Management controversies. Pediatr Nephrol. 2004;19:819-20.
- [Google Scholar]
- Conservative treatment of ureteropelvic junction obstruction in children with antenatal diagnosis of hydronephrosis: Lessons learned after 16 years of follow-up. Eur Urol. 2006;49:734-8.
- [Google Scholar]
- The sonographic diagnosis of infravesical obstruction in children: Evaluation of bladder wall thickness indexed to bladder filling. J Urol. 1997;157:989-91.
- [Google Scholar]
- Primary vesicoureteric reflux - How useful is postnatal ultrasound? Arch Dis Child. 1996;75:444-7.
- [Google Scholar]
- Posterior urethral valves: Multivariate analysis of factors affecting the final renal outcome. J Urol. 2011;185:2491-5.
- [Google Scholar]
- Renal pyramid echogenicity in ureteropelvic junction obstruction: Correlation between altered echogenicity and differential renal function. Pediatr Radiol. 2008;38:1068-73.
- [Google Scholar]
- Increased echogenicity as a predictor of poor renal function in children with grade 3-4 hydronephrosis. J Urol. 2006;175:1898-901.
- [Google Scholar]
- Antenatal hydronephrosis: Infants with minor postnatal dilatation do not need prophylaxis. Pediatr Nephrol. 2008;23:2021-4.
- [Google Scholar]
- Prenatally detected primary megaureter: A role for extended follow-up. J Urol. 2005;173:1353-6.
- [Google Scholar]
- Late recurrence of symptomatic hydronephrosis in patients with prenatally detected hydronephrosis and spontaneous improvement. J Urol. 2008;180:322-5.
- [Google Scholar]
- Antenatal hydronephrosis with postnatal resolution: How long are postnatal studies warranted? Urology. 2001;57:1178.
- [Google Scholar]
- Analysis of trends on serial ultrasound for high grade neonatal hydronephrosis. J Urol. 2002;168:1518-21.
- [Google Scholar]
- Do infants with mild prenatal hydronephrosis benefit from screening for vesicoureteral reflux? J Urol. 2012;188:576-81.
- [Google Scholar]
- Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol. 2010;184:1134-44.
- [Google Scholar]
- Applying the ALARA concept to the evaluation of vesicoureteric reflux. Pediatr Radiol. 2006;36:185-91.
- [Google Scholar]
- Outcome of isolated antenatal hydronephrosis: A prospective cohort study. Pediatr Nephrol. 2007;22:1727-34.
- [Google Scholar]
- Short-term outcome of mild isolated antenatal hydronephrosis conservatively managed. J Pediatr Urol. 2012;8:129-33.
- [Google Scholar]
- Mild fetal renal pelvis dilatation: Much ado about nothing? Clin J Am Soc Nephrol. 2009;4:168-77.
- [Google Scholar]
- Imaging recommendations in paediatric uroradiology: Minutes of the ESPR workgroup session on urinary tract infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography, Barcelona, Spain, June 2007. Pediatr Radiol. 2008;38:138-45.
- [Google Scholar]
- Incidental vesicoureteral reflux in neonates with antenatally detected hydronephrosis and other renal abnormalities. Radiology. 1993;187:157-60.
- [Google Scholar]
- Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. 2007;18:CD001532.
- [Google Scholar]
- Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: A multicenter, randomized, controlled study. Pediatrics. 2006;117:626-32.
- [Google Scholar]
- Vesicoureteral reflux in infants with isolated antenatal hydronephrosis. Pediatr Nephrol. 2003;18:1224-8.
- [Google Scholar]
- Renal sonography is not a reliable screening examination for vesicoureteral reflux. J Urol. 1993;150:752-5.
- [Google Scholar]
- Antenatal and postnatal ultrasound in the evaluation of the risk of vesicoureteral reflux. Pediatr Nephrol. 2010;25:1687-92.
- [Google Scholar]
- Does hydronephrosis predict the presence of severe vesicoureteral reflux? Eur J Pediatr. 2012;171:1605-10.
- [Google Scholar]
- Subcommittee on Urinary Tract Infection. Technical report-Diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128:e749-70.
- [Google Scholar]
- Brussels Free University Perinatal Nephrology (BFUPN) Study Group. Results of systematic voiding cystourethrography in infants with antenatally diagnosed renal pelvis dilation. J Pediatr. 2002;141:21-4.
- [Google Scholar]
- Patient dose reduction during voiding cystourethrography. Pediatr Radiol. 2006;36 Suppl 2:168-72.
- [Google Scholar]
- Children and adolescents with ureteropelvic junction obstruction: Is an additional voiding cystourethrogram necessary? Results of a multicenter study. World J Urol 2012 [Epub ahead of print]
- [Google Scholar]
- Auspices of Paediatric Committee of European Association of Nuclear Medicine. Guidelines for standard and diuretic renogram in children. Eur J Nucl Med Mol Imaging. 2011;38:1175-88.
- [Google Scholar]
- 99mTc-MAG3: Review of pharmacokinetics, clinical application to renal diseases and quantification of renal function. Ann Nucl Med. 2001;15:179-90.
- [Google Scholar]
- 99mTc ethylene dicysteine scintigraphy for diagnosing cortical defects in acute pyelonephritis: A comparative study with 99m Tc dimercaptosuccinic acid. Nucl Med Commun. 2004;25:967-70.
- [Google Scholar]
- EANM Dosimetry and Paediatrics Committees. The new EANM paediatric dosage card. Eur J Nucl Med Mol Imaging. 2008;35:1748.
- [Google Scholar]
- 99mTc-MAG3 diuretic renography in children: A comparison between F0 and F+20. Nucl Med Commun. 2003;24:1189-93.
- [Google Scholar]
- The well tempered diuretic renogram: A standard method to examine the asymptomatic neonate with hydronephrosis or hydroureteronephrosis.A report from combined meetings of The Society for Fetal Urology and members of The Pediatric Nuclear Medicine Council-The Society of Nuclear Medicine. J Nucl Med. 1992;33:2047-51.
- [Google Scholar]
- Antenatal detection of pelviureteric junction stenosis: Main controversies. Semin Nucl Med. 2011;41:11-9.
- [Google Scholar]
- Consensus report on quality control of quantitative measurements of renal function obtained from the renogram: International Consensus Committee from the Scientific Committee of Radionuclides in Nephrourology. Semin Nucl Med. 1999;29:146-59.
- [Google Scholar]
- Interpretation of the renogram: Problems and pitfalls in hydronephrosis in children. BJU Int. 2004;94:887-92.
- [Google Scholar]
- Antenatally detected, unilateral dilatation of the renal pelvis: A critical review. 1. Postnatal non-operative treatment 20 years on– Is it safe Scand? J Urol Nephrol. 2002;36:243-50.
- [Google Scholar]
- Value of supranormal function and renogram patterns on 99mTc-mercaptoacetyltriglycine scintigraphy in relation to the extent of hydronephrosis for predicting ureteropelvic junction obstruction in the newborn. J Nucl Med. 2003;44:725-31.
- [Google Scholar]
- Supranormal differential renal function is real but may be pathological: Assessment by 99 m technetium mercaptoacetyltriglycine renal scan of congenital unilateral hydronephrosis. J Urol. 2001;165:2300-4.
- [Google Scholar]
- Impaired drainage on diuretic renography using half-time or pelvic excretion efficiency is not a sign of obstruction in children with a prenatal diagnosis of unilateral renal pelvic dilatation. J Urol. 2003;169:1828-31.
- [Google Scholar]
- Current trends in the management of posterior urethral valves in the pediatric population. Urology. 2002;60:947-53.
- [Google Scholar]
- Long-term outcome of prenatally detected posterior urethral valves: Single center study of 65 cases managed by primary valve ablation. J Urol. 2008;179:307-12.
- [Google Scholar]
- Factors affecting outcome in the management of posterior urethral valves. Pediatr Surg Int. 2001;17:11-5.
- [Google Scholar]
- Outcome analysis of pediatric pyeloplasty as a function of patient age, presentation and differential renal function. J Urol. 1995;154:1889-93.
- [Google Scholar]
- Antenatally detected pelviureteric junction obstruction. Is non-operation safe? Br J Urol. 1991;68:305-10.
- [Google Scholar]
- Prenatal diagnosis: What do we know of long-term outcomes? J Pediatr Urol. 2010;6:204-11.
- [Google Scholar]
- Plasma renin activity for monitoring vesicoureteric reflux therapy: Mid-term observations. J Pediatr Urol. 2008;4:60-4.
- [Google Scholar]
- Operative versus nonoperative management of ureteropelvic junction obstruction in children. Urology. 2009;73:521-5.
- [Google Scholar]
- The postnatal management of hydronephrosis diagnosed by prenatal ultrasound. J Urol. 1990;144:584-7.
- [Google Scholar]
- Progression of hydronephrosis correlates with worsening renal function in children. Conference: 2009 American Urological Association (AUA) Annual Meeting Chicago, IL United States. Conference Publication. J Urol. 2009;181:443.
- [Google Scholar]
- Unilateral hydronephrosis due to ureteropelvic junction obstruction in children: Long term follow-up. Minerva Urol Nefrol. 2009;61:325-9.
- [Google Scholar]
- Does delaying pyeloplasty affect renal function in children with a prenatal diagnosis of pelvi-ureteric junction obstruction? BJU Int. 2002;90:72-5.
- [Google Scholar]
- Clinical and radiological characteristics of patients operated in the first year of life due to ureteropelvic junction obstruction: Significance of renal pelvis diameter. Urology. 2009;74:898-902.
- [Google Scholar]
- Postnatal outcome of prenatally diagnosed severe fetal renal pelvic dilatation. Prenat Diagn. 2012;32:519-22.
- [Google Scholar]
- Prenatally diagnosed hydronephrosis: The Great Ormond Street experience. Br J Urol. 1998;81:39-44.
- [Google Scholar]
- The nonoperative management of unilateral neonatal hydronephrosis: Natural history of poorly functioning kidneys. J Urol. 1994;152:593-5.
- [Google Scholar]
- Comparison between unilateral pyeloplasty and conservative treatment in bilateral ureteropelvic junction obstruction of children. Korean J Urol. 1998;39:1248-53.
- [Google Scholar]
- Long-term followup of prenatally detected severe bilateral newborn hydronephrosis initially managed nonoperatively. J Urol. 2002;168:1118-20.
- [Google Scholar]
- Management of severe bilateral ureteropelvic junction obstruction in neonates with prenatally diagnosed bilateral hydronephrosis. Korean J Urol. 2010;51:653-6.
- [Google Scholar]
- Nonoperative management of neonatal moderate to severe bilateral hydronephrosis. J Urol. 2002;167:662-5.
- [Google Scholar]
- Vesicoureteral reflux and urinary tract infection in children with a history of prenatal hydronephrosis-should voiding cystourethrography be performed in cases of postnatally persistent grade II hydronephrosis? J Urol. 2009;181:801-6.
- [Google Scholar]
- Nonrefluxing neonatal hydronephrosis and the risk of urinary tract infection. J Urol. 2008;179:1524-8.
- [Google Scholar]
- Is antibiotic prophylaxis necessary in infants with obstructive hydronephrosis? J Urol. 2007;177:1098-101.
- [Google Scholar]
- Postnatal assessment of growth, nutrition, and urinary tract infections of infants with antenatally detected hydronephrosis. Int Urol Nephrol. 2010;42:781-8.
- [Google Scholar]
- Antibiotic prophylaxis for urinary tract infections in antenatal hydronephrosis. Pediatrics. 2013;131:e251-61.
- [Google Scholar]
- Antibiotic prophylaxis for children at risk of developing urinary tract infection: A systematic review. Acta Paediatr. 2009;98:1781-6.
- [Google Scholar]
- Evidence for and against urinary prophylaxis in vesicoureteral reflux. Pediatr Nephrol. 2010;25:2379-82.
- [Google Scholar]
- Revised statement on management of urinary tract infections. Indian Pediatr. 2011;48:709-17.
- [Google Scholar]
- Renal parenchymal damage in male infants with high grade vesicoureteral reflux diagnosed after the first urinary tract infection. J Urol. 2002;168:1708-10.
- [Google Scholar]
- Current management of infants with fetal renal pelvis dilation: A survey by French-speaking pediatric nephrologists and urologists. Pediatr Nephrol. 2004;19:966-71.
- [Google Scholar]
- Probability of urinary tract infection in infants with ureteropelvic junction obstruction: Is antibacterial prophylaxis really needed? Pediatr Nephrol. 2011;26:1837-41.
- [Google Scholar]
- Occurrence of urinary tract infection in children with significant upper urinary tract obstruction. Urology. 2009;73:74-8.
- [Google Scholar]
- Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277-84.
- [Google Scholar]
- Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology. 2008;248:254-63.
- [Google Scholar]
- An update of radiopharmaceutical schedules in children. Nucl Med Commun. 1998;19:1023-36.
- [Google Scholar]
- Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet. 2012;380:499-505.
- [Google Scholar]
- Minimizing and communicating radiation risk in pediatric nuclear medicine. J Nucl Med Technol. 2012;40:13-24.
- [Google Scholar]







