Translate this page into:
Pulmonary histoplasmosis in a renal allograft recipient
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Histoplasmosis, seen rarely in kidney transplantracipients, can vary from an innocuous illness often misdiagnosed as tuberculosis to a severe disseminated disease with a high mortality. We describe a case with non-specific signs in whom the diagnosis was made by histopathological examination of the lesion. Prompt introduction of specific treatment led to Histoplasmosis, seen rarely in kidney transplantracipients, can vary from an innocuous illness often misdiagnosed as tuberculosis to a severe disseminated disease with a high mortality. We describe a case with non-specific signs in whom the diagnosis was made by histopathological examination of the lesion. Prompt introduction of specific treatment led to the patient making an excellent recovery the patient making an excellent recovery.
Keywords
Histoplasmosis
intracellular yeasts
renal allograft recipient
Introduction
Histoplasmosis reported from the Gangetic plains in India,[12] is rare in renal transplantation,[23] and more common in human immunodeficiency virus (HIV) infected individuals. As the presenting clinical features are usually prolonged fever, malaise, weight loss, cough, dyspnea and an abnormal chest roentgenogram with diffuse nodular patchy or miliary infiltrates,[4] the condition may be misdiagnosed as tuberculosis and has been empirically treated as such without definitive investigations.[1] Here, we report a case of pulmonary histoplasmosis developing in a renal allograft recipient in a non-endemic area with no documented exposure.
Case Report
A 55-year-old lady, on dialysis since February 2011, underwent a renal transplantation on 23rd February 2012 with an allograft kidney from her daughter. She had been diagnosed with tuberculous lymphadenopathy in September 2011 and received isoniazid, rifampicin, pyrazinamide and ethambutol from September to December 2011. Pyrazinamide was stopped in December 2011 and rifampicin replaced with ciprofloxacin in February 2012. There was 1 B and 1 DR mismatch and the lymphocyte cross match by complement dependent cytotoxicity was negative at 4%.
Immunosuppression consisted of intraoperative methylprednisolone, followed by tapering doses of oral prednisolone until 10 mg daily, tacrolimus 0.15 mg/kg/day adjusted according to trough levels and azathioprine 2 mg/kg/day.
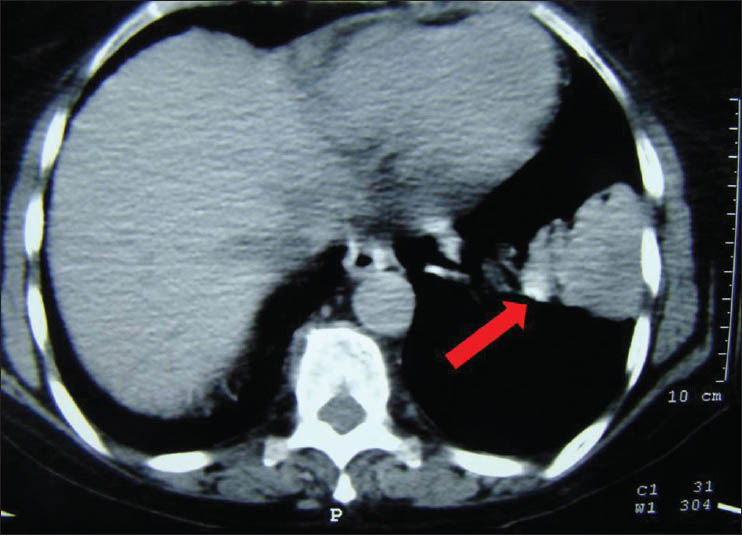
She was admitted on 18th July 2012 with daily evening fever up to 100°F for 10 days, dry cough for 7 days and left sided pleuritic chest pain for 5 days. She had received oral amoxicillin clavulunate and paracetamol with temporary relief, but symptoms had worsened on the day of admission. Examination revealed a temperature of 100.4°F, pulse of 96/min and respiratory rate of 20/min. Blood pressure was 130/85 mm of Hg. Auscultation of the chest revealed decreased breath sounds at the left infraaxillary and infrascapular regions, without any bronchial breathing, rub or rales. A chest roentgenogram showed a homogeneous opacity without an air bronchogram in the left lower zone with obscured left costophrenic angle [Figure 1].

- Chest X-ray at presentation shows opacity in left hemithorax
Other investigations are shown in Table 1.
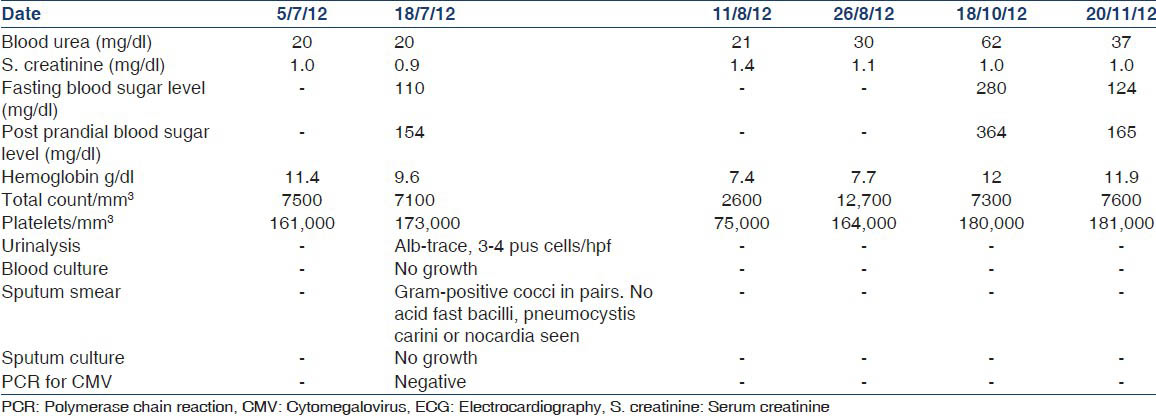
She received empirical cefotaxime 2 g q 12 h, azithromycin 0.5 g daily and nebulization to induce sputum production. She continued to have chest pain and fever despite negative blood cultures and a complete absence of sputum. A left lateral roentgenogram confirmed the opacity and showed no evidence of pleural fluid. A high resolution computed tomography scan of the thorax on the 25th of July 2012, revealed a heterogeneously enhancing necrotic mass lesion in the left lower lateral basal segment reaching up to the pleural surface, multiple discrete lower lobe nodules bilaterally, a discrete 1 cm nodular density in the inferior and paracardiac region of the right upper lobe and enlarged pre-tracheal, paratracheal, precarinal and subcarinal lymph nodes [Figure 2].
- High resolution computed tomography thorax. Arrow shows inhomogeneous mass in left hemithorax, non-enhancing areas indicate necrosis
A fine-needle aspiration and biopsy of the lesion was performed on 26th July 2012, under computed tomographic guidance. The aspirate showed a necrotic background with few polymorphs, lymphocytes, eosinophils and budding yeast like fungal elements and was negative for acid fast bacilli, nocardia and Pneumocystis jiroveci. The biopsy showed widespread areas of necrosis, infiltration with polymorhonuclear leukocytes and lymphocytes and histiocytic proliferation and fibrosis. The histiocytes contained multiple tiny, budding yeast like structures, which on Periodic acid Schiff (PAS), showed a distinct positively stained capsule [Figure 3]. The lesion was suggestive of histoplasmosis, which was confirmed on culture after 23 days of incubation. Patient was treated with liposomal amphotericin B 200 mg daily, (4 mg/kg/day) and discontinuation of azathioprine. After 20 days, her chest roentgenogram showed significant improvement and she was discharged on oral itraconazole 200 mg bid and her chest roentgenogram after 5 months of treatment [Figure 4], showed complete resolution of the lesion. In October 2012, she was found to have developed new onset diabetes after transplantation, which was controlled with oral hypoglycemic agents.

- Gomoris silver stain showing typical budding yeasts (arrow head) against a necrotic background, ×1000
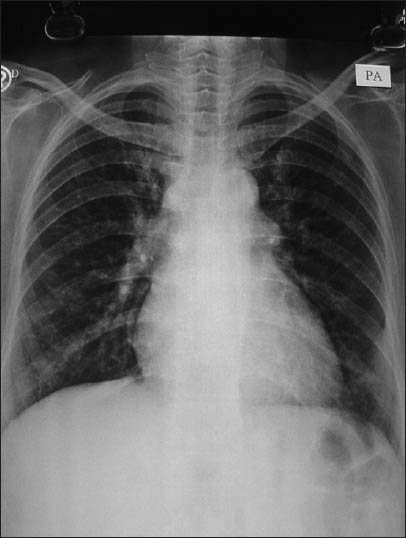
- Chest X-ray showing resolution after 5 months of treatment
Discussion
Histoplasmosis despite its world-wide distribution, has been uncommonly reported from India.[12] It is rare in renal transplantation, usually does not appear to be related to the intensity of immunosuppression and can occur at any time,[23] with the world's largest series reporting an attack rate of 0.6/1000 patient years over a 10 year follow-up period.[4] Immunosuppressed patients may develop disseminated disease,[35] which can progress to septic shock, disseminated intravascular coagulation, acute kidney injury and respiratory insufficiency[24] and carries a mortality of up to 23%.[6] The presenting clinical features, usually prolonged fever, malaise, weight loss, cough, dyspnea and an abnormal chest roentgenogram with diffuse nodular patchy or miliary infiltrates,[4] resemble tuberculosis, which is endemic in India and hence it has been empirically treated as such without definitive investigations.[1] Falsely negative serological tests in immunosuppressed patients and negative antigen tests in patients without complete disseminated disease, make distinguishing the condition from other fungal infections, tuberculosis, sarcoidosis, lymphoma and malignancy difficult or impossible in the patient with pyrexia of unknown origin, without histopathological examination.[6] The largest Indian series of 24 cases reported diabetes and HIV infection to be the most common predisposing features with only one patient a renal allograft recipient.[1] The most common presentation was subacute and ten patients had received empirical anti-tuberculous treatment for 2-3 months prior to diagnosis.
Additional case reports from India specifically in renal allograft recipients have reported cutaneous,[6] pulmonary,[3] concomitant colonic and genitourinary histoplasmosis presenting as a prostatic abscess[7] and disseminated forms[2] at different time periods.
The organism is a dimorphic fungus, present as a mold in its natural reservoir, soil contaminated with bird droppings and converts to a budding yeast form in tissues during the invasive disease. Acute pulmonary disease usually an asymptomatic disease can become severe and develop into acute respiratory distress syndrome in patients who have defective cell mediated immunity, while the untreated chronic form progresses to increasing pulmonary insufficiency and death.[8] The presence of systemic signs such as fever (seen in 93% of organ recipients),[4] malaise, weight loss and evidence of bone marrow suppression indicate dissemination and is an indication for urgent treatment especially in solid organ recipients.[8] In our patient, these signs developed while she had already been treated for tuberculosis for 10 months.
The diagnosis rests upon histopathology of affected areas most often with demonstration of intracellular yeasts, the presence of budding and a distinct capsule seen best on the PAS or Gomori's Silver stain, all of which were seen in our patient. The value of direct demonstration of the organisms on smear or tissue was stressed in two studies both of which found the sensitivity to be 100%,[19] in contrast to culture, which was positive in 20.8%[1] and 16%[9] respectively. As in our case, all studies reporting positive cultures noted a period of 3-4 weeks on special media to develop the mold form with the presence of microconidia.[168] The absence of granuloma formation and presence of necrosis are associated with progressive disease and this prompted us to start treatment with liposomal amphotericin B, as per the American Thoracic Society recommendations[10] before confirmation of the species Histoplasma capsulatum on culture. Most Indian reports show similar patterns, as the serology maybe negative in immunosuppressed individuals and the mortality has been decreased from 80% to <25% with antifungals.[110] Our patient made a complete recovery with clinical cure and radiological resolution and was maintained on oral itraconazole therapy.
Conclusion
As stated by Gopalakrishnan et al.,[1] histoplasmosis may be underdiagnosed in India as it requires a high index of clinical suspicion and tissue diagnosis and growth on culture may be late. Treatment with amphotericin B and itraconazole is associated with a good outcome.
Acknowledgments
The authors gratefully thank Drs. K. B. Niphadkar, Sonali Sanghvi, Meenakshi Satpute and N. V. Telang of the Department of Microbiology for their excellent support in this and all our difficult cases.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Histoplasmosis in India: Truly uncommon or uncommonly recognised? J Assoc Physicians India. 2012;60:25-8.
- [Google Scholar]
- Disseminated histoplasmosis 19 years after renal transplantation. Clin Nephrol. 1999;51:373-8.
- [Google Scholar]
- Renal allograft recipient with co-existing BK virus nephropathy and pulmonary histoplasmosis: Report of a case. Clin Exp Nephrol. 2010;14:641-4.
- [Google Scholar]
- Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis. 2009;49:710-6.
- [Google Scholar]
- Disseminated histoplasmosis in a renal transplant recipient – A fatal combination. J Med. 2011;12:163-5.
- [Google Scholar]
- Infection with Histoplasma capsulatum in a renal transplant recipient. Saudi J Kidney Dis Transpl. 2010;21:1115-7.
- [Google Scholar]
- Genitourinary histoplasmosis in post-renal transplant patient: Diagnostic dilemma. Indian J Urol. 2012;28:359-61.
- [Google Scholar]
- Histoplasmosis: A clinical and laboratory update. Clin Microbiol Rev. 2007;20:115-32.
- [Google Scholar]
- An official American thoracic society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96-128.
- [Google Scholar]







