Translate this page into:
Nitric oxide status in patients with chronic kidney disease
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Patients with chronic kidney disease (CKD) are at an increased risk of cardiovascular (CVD) morbidity and mortality, mainly due to atherosclerosis. Decreased production or reduced bioavailability of nitric oxide (NO) can result in endothelial dysfunction (ED). Multiple mechanisms are known to cause a state of NO deficiency in patients with CKD. Patients in various stages of CKD grouped as group-1 (CKD stage 1 and 2), group-2 (CKD stage 3 and 4), group-3 (CKD stage 5) and healthy controls were included in the study. Each group of patients and controls comprised 25 subjects. Plasma nitrites, L-arginine, asymmetric dimethyl arginine (ADMA) and citrulline were measured in all the subjects. Patients in all stages of CKD had lower NO and higher ADMA levels compared to controls. Further, group-2 and group-3 patients had lower levels of NO and higher levels of ADMA than group-1 patients. L-arginine levels showed no difference between patients and controls. However, group-3 patients had lower L-arginine levels compared to group-1 patients. Citrulline levels were decreased in group-3 patients. NO production was decreased in patients in all stages of CKD. The decrease could be due to decreased availability of the substrate, L-arginine or due to an increased ADMA, a potent inhibitor of endothelial NO synthase. Therapeutic interventions directed towards improvement of NO production in addition to management of other CVD risk factors may prevent development of ED and facilitate proper management of CKD patients who are at increased risk for CVD.
Keywords
Asymmetric dimethyl arginine
chronic kidney disease
citrulline
endothelial dysfunction
nitric oxide
Introduction
Chronic kidney disease (CKD) represents a world-wide public health problem. CKD is associated with an increased prevalence of cardiovascular (CVD) disease and the relationship between renal dysfunction and adverse CVD events is now well established.[12] It has been proposed that endothelial dysfunction (ED), which is an early and critical event in atherosclerosis is the principal pathophysiological mechanism that provides an important link between renal disease and the increased risk of CVD present in patients with CKD. A combination of traditional and non-traditional CVD risk factors that can affect endothelial function have been found in patients with CKD.[3] ED has a complex pathophysiology involving multiple mechanisms.[4] Either reduced synthesis or decreased bioavailability of Nitric oxide (NO) is one of the fundamental mechanisms in the development of ED.[5]
Earlier studies have reported that NO production is decreased in CKD[67] and multiple mechanisms were found to be involved in causing NO deficiency in these patients.[7] NO is synthesized from L-arginine by endothelial NO synthase (e-NOS), which incorporates molecular oxygen into the guanidino group of L-arginine, yielding NO and L-citrulline as products.[6] Deficiency of L-arginine, increase in endogenous NOS inhibitors, decrease in the activity of NOS and reduced availability of essential cofactors can result in decreased NO synthesis.[7] Asymmetric dimethyl arginine (ADMA), a potent endogenous competitive inhibitor of NOS, was found to be associated with ED.[4] Studies have shown that plasma ADMA levels were increased in patients with renal disease.[89]
Since ED is an early event in the process of atherogenesis,[4] understanding the mechanisms that impair endothelial function in patients with CKD may help to devise effective strategies to reduce the CVD risk that is highly prevalent in them.[10] In this background, the present study was conducted to study ED across the whole range of renal function.
Subjects and Methods
Subjects
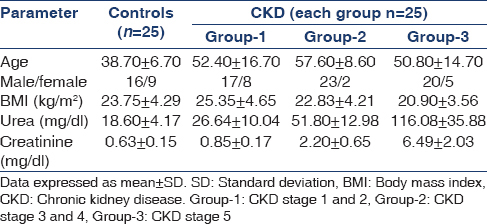
The present study included patients in various stages of CKD attending the out-patient department of Nephrology in a tertiary care center and non-smoking, non-diabetic and normotensive healthy subjects as controls. Patients with CKD stage 1 and 2 were included in group-1, CKD stage 3 and 4 in group-2 and CKD stage 5 in group-3. Controls and each group of CKD patients comprised 25 subjects.
Chronic kidney disease was defined as per Kidney Disease Initiatives and Global Outcome guidelines.[11] Glomerular filtration rate (GFR) was calculated using the Cockcroft-Gault equation.[12] Patients with acute renal failure, acute on CKD, H/O smoking, congestive heart failure, current pregnancy were excluded from the study. The study was approved by institutional Ethics Committee.
Five milliliter of venous blood was collected in heparinized tubes from all the participants after an informed consent. Plasma was separated by centrifugation and stored at −80°C until further analysis. NO measured as nitrites was estimated by Griess method[13] using Perkin Elmer Lambda 1.2 ultraviolet Vis double beam spectrophotometer; ADMA, L-arginine and citrulline were measured by high performance liquid chromatography (HPLC).[14] Chromatographic analysis was done using a Shimadzu (Kyoto, Japan) LC-20A HPLC system, and RF-10 AXL fluorescence detector and a C-R6A chromatopac data processor. Separation was performed using a 150 mm × 4.6 mm Novapak C 18 column with a particle size of 5 μ protected by a C18 guard-pak cartridge.
Continuous variables were expressed as mean ± standard deviation. Comparison between the groups was done using one way analysis of variance followed by post-hoc Bonferroni test for multiple comparisons. The association between NO, arginine, ADMA and citrulline with serum creatinine and estimated GFR (eGFR) was analyzed using Pearson's correlation. Analysis was done on Statistical Package for the Social Sciences version 11.5 (SPSS, Inc., Chicago IL). A P < 0.05 was considered statistically significant.
Results
The demographic characteristics of controls and CKD patients were shown in Table 1. Plasma levels of NO, L-arginine, ADMA, citrulline, L-arginine/citrulline ratio and L-arginine/ADMA ratio in controls and patients with CKD were shown in Table 2 and Figure 1. The statistical significance of the direction of change in the parameters studied was shown in Table 3.


- The trend of changes in the parameters studied with progression of chronic kidney disease

Patients in all stages of CKD had significantly lower plasma NO levels when compared to controls. The levels decreased with increasing renal failure, with group-2 and group-3 patients having significantly lower levels than group-1 patients. L-arginine levels in the present study showed no significant difference between patients and controls. However, L-arginine in patients showed a decreasing trend that became statistically significant in group-3 consisting of patients in CKD stage 5. ADMA levels were found to be significantly increased in all patients with CKD when compared to controls. Further, the levels showed an increasing trend with advancing renal disease, with group-2 and group-3 patients having significantly higher levels when compared to patients in early stages of CKD (group-1). Although plasma citrulline levels showed no significant difference across all the groups, the levels decreased with progression of CKD reaching statistically significant levels in group-3 patients when compared to controls, group-1 and group-2 CKD patients.
L-arginine/citrulline ratio was found to be significantly higher in group-2 and group-3 patients when compared to controls. The ratio showed an increasing trend in patients with CKD as evidenced by significantly higher ratio in patients with CKD stage 5 (group-3) when compared to patients in early stages of CKD (group-1). All patients with CKD had significantly lower L-arginine/ADMA ratio than controls. The ratio decreased with increasing renal failure, with group-2 and group-3 CKD patients showing significantly lower ratio than group-1 patients.
Nitric oxide showed significant positive correlation with eGFR (r = 0.476, P = 0.016) in patients with stage 3 and 4 CKD; and citrulline showed significant positive correlation with creatinine in patients with stage 1 and 2 CKD (r = 0.415, P = 0.044). The association between all the other parameters studied was found to be not significant.
Discussion
Plasma NO levels in the present study were found to be significantly lower in all stages of CKD patients when compared to controls. This is in line with the finding of Wever et al.[6] who demonstrated that basal whole body NO production is reduced in patients with chronic renal failure. Decreased NO observed in our study may reflect ED that has been reported in patients with CKD even in the early stages of the disease.[15] Multiple mechanisms can result in NO deficiency in patients with CKD[7] and include decreased availability of the substrate for NOS, L-arginine and increased ADMA, an inhibitor of NOS. In the present study, no significant difference was observed in L-arginine levels between patients and controls. Kielstein et al.[8] and Schmidt and Baylis[16] in their studies also reported similar L-arginine concentrations in controls and patients with renal disease. However, the L-arginine levels in the present study decreased by about 25% in patients with CKD stage 5 when compared to healthy controls and also, the levels showed a significant decrease in this group of patients when compared to group-1 patients, indicating decreased availability of substrate. Renal production of L-arginine is the major endogenous source of arginine to the body. Patients with advanced CKD or end-stage renal disease, who have a reduced normal functional renal tissue are expected to have a decreased production of L-arginine. However, the relationship between the amount of residual renal function and arginine biosynthetic capacity is yet to be known fully.[7]
Plasma levels of ADMA in patients in all stages of CKD in the present study were found to be significantly higher than controls. Kielstein et al.[8] and Schmidt and Baylis[16] also reported elevated ADMA levels in patients with chronic renal disease when compared to controls. Also, in the present study, plasma levels of ADMA increased with decrease in renal function. The levels increased through various stages of CKD, with group-2 and group-3 patients having significantly higher levels than group-1 patients. The increase was found to be not correlating with renal dysfunction estimated as serum creatinine or eGFR in any of the groups studied. This is in line with Kielstein et al. and Schmidt and Baylis who also have not observed significant correlation between ADMA levels and GFR[8] or creatinine values.[16] Increased ADMA could be a result of increased methylation of arginine due to increased activity of protein arginine methyl transferase[17] or decreased metabolism due to decreased activity of the enzyme dimethyl arginine dimethyl amino hydrolase.[81617]
Asymmetric dimethyl arginine is now being recognized as an important biomarker of atherosclerosis.[18] The competitive inhibition of e-NOS binding to L-arginine by ADMA has been considered to be responsible for decreased NO production and induction of ED. However, recent studies suggest that ADMA inhibition of NO production occurs by mechanisms independent of substrate inhibition.[19] Zoccali et al.[20] reported plasma concentration of ADMA as the second strongest predictor of mortality and CVD in patients with chronic renal failure. Thus, elevated plasma ADMA levels increase CVD risk through reduced NO production, which can cause ED, hypertension and proatherosclerotic changes and through accelerating senescence of endothelial cells.[21]
Plasma citrulline levels were found to be significantly decreased in group-3 patients when compared to controls, group-1 and group-2 patients in the present study. Earlier studies have reported increased citrulline levels in CKD patients compared to controls.[1622] Citrulline is converted in the body to arginine through the arginino succinate pathway. In patients with CKD, a decreased renal uptake of citrulline and its conversion to arginine can result in elevated plasma citrulline levels. On the other hand, NOS converts arginine to NO and citrulline.[23] Small intestine is the major source of citrulline in serum and decreased citrulline levels were reported in patients with intestinal disease.[24] However, none of the patients in the present study had intestinal disease to explain the decrease in citrulline levels. Ware et al.[25] reported that extracellular citrulline is an effective precursor of NO production in human vascular endothelial cells, thus suggesting that circulating levels of citrulline may be more predictive of NOS function than arginine levels. Hence, the decrease found in our study might reflect decreased activity of e-NOS and may be considered as an indicator of decreased NO production.
The arginine/citrulline ratio in the present study was found to be significantly high in group-2 and group-3 patients compared to controls. The ratio showed an increasing trend in CKD patients, with group-3 patients having significantly higher ratio than group-1 patients.
Arginine/ADMA ratio was significantly decreased in all patients with CKD when compared to controls in the present study. The decrease was more pronounced in patients with advancing renal disease, with group-2 and group-3 patients having significantly lower ratio than group-1 patients. Kielstein et al.[8] also reported markedly decreased L-arginine/ADMA ratio in patients with renal disease. A ratio of L-arginine and ADMA is considered to determine cell-specific L-arginine uptake and NO generation in renal tubular cells as well as endothelial cells.[8] Thus, L-arginine/ADMA ratio is considered to represent bioavailability of NO[23] and a decreased L-arginine/ADMA ratio was reported to be associated with atherosclerosis.[26]
Conclusion
Results of the present study show that NO production is decreased in patients with renal failure, which is understandably one of the major factors causing ED in these patients. Decreased NO production may be due to decreased availability of the substrate arginine or an increased accumulation of ADMA, which is a potent inhibitor of the enzyme e-NOS. We have observed both decreased levels of substrate as well as increase in ADMA levels in the present study and decreased production of NO is further supported by decrease in citrulline levels. Also, increased ADMA levels are known to have a direct effect on increasing CVD risk. Thus, our findings point toward the presence of CVD risk even in the early stages of CKD that further increases with the progression of the disease.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension. 2003;42:1050-65.
- [Google Scholar]
- Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol. 2006;17:2034-47.
- [Google Scholar]
- Chronic kidney disease: Effects on the cardiovascular system. Circulation. 2007;116:85-97.
- [Google Scholar]
- Effect of a single hemodialysis session on endothelial dysfunction. J Nephrol. 2011;24:83-90.
- [Google Scholar]
- Nitric oxide production is reduced in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 1999;19:1168-72.
- [Google Scholar]
- Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294:F1-9.
- [Google Scholar]
- Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J Am Soc Nephrol. 2002;13:170-6.
- [Google Scholar]
- Impact of vitamin E on plasma asymmetric dimethylarginine (ADMA) in chronic kidney disease (CKD): A pilot study. Nephrol Dial Transplant. 2003;18:2415-20.
- [Google Scholar]
- Peripheral vascular dysfunction in chronic kidney disease. Cardiol Res Pract. 2011;2011:267257.
- [Google Scholar]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266.
- [Google Scholar]
- Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440-3.
- [Google Scholar]
- High performance liquid chromatographic determination of amino acids in biological samples by precolumn derivation with O-phthaldialdehyde. J Liq Chromatogr. 1987;10:941-55.
- [Google Scholar]
- Abnormalities of endothelial function in patients with predialysis renal failure. Heart. 2000;83:205-9.
- [Google Scholar]
- Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000;58:1261-6.
- [Google Scholar]
- Molecular mechanism for elevation of asymmetric dimethylarginine and its role for hypertension in chronic kidney disease. J Am Soc Nephrol. 2006;17:2176-83.
- [Google Scholar]
- ADMA and NOS regulation in chronic renal disease: Beyond the old rivalry for l-arginine. Kidney Int. 2012;81:722-4.
- [Google Scholar]
- Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet. 2001;358:2113-7.
- [Google Scholar]
- Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat Clin Pract Nephrol. 2006;2:209-20.
- [Google Scholar]
- Plasma and urinary amino acid metabolomic profiling in patients with different levels of kidney function. Clin J Am Soc Nephrol. 2014;9:37-45.
- [Google Scholar]
- High citrulline-to-arginine ratio associated with blood pressure abnormalities in children with early chronic kidney disease. Circ J. 2013;77:181-7.
- [Google Scholar]
- Biomarkers for radiation-induced small bowel epithelial damage: An emerging role for plasma Citrulline. World J Gastroenterol. 2007;13:3033-42.
- [Google Scholar]
- Low plasma citrulline levels are associated with acute respiratory distress syndrome in patients with severe sepsis. Crit Care. 2013;17:R10.
- [Google Scholar]
- Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation. 1997;95:2068-74.
- [Google Scholar]







