Translate this page into:
Role of 24-h ambulatory blood pressure monitoring in children with chronic kidney disease
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hypertension is common in children with chronic kidney disease (CKD) and is a major determinant of CKD progression. Ambulatory blood pressure monitoring (ABPM) has been proposed to be better in detecting hypertension as compared to casual blood pressure (CBP). This study aims to study the usefulness of ABPM in detecting masked hypertension, evaluating the adequacy of blood pressure (BP) control and predicting left ventricular hypertrophy (LVH) amongst children with CKD. A prospective cross-sectional study of 46 children with stage 3–5 CKD was conducted at the Pediatric Nephrology department of a tertiary hospital in South India. All children underwent CBP, ABPM and an echocardiography. Results were categorized as normal BP; confirmed hypertension; masked hypertension and white coat hypertension. Out of 46 children studied, 11 were undergoing dialysis. While 39.1% children had stage 3 and 4 CKD each, 21.7% had stage 5 CKD. Masked hypertension was detected in 19.6% and 21.7% had confirmed hypertension. Thirty-four (73.9%) children were already receiving antihypertensive medication. In these, CBP was elevated in 23.5% and ABP in 47%. Among children with hypertension as defined by ABPM, LVH was detected in 32.2%. We found that higher the number of abnormal ABPM indices (assessed by BP Index, nocturnal dipping and BP Load) higher the likelihood of LVH (P = 0.046). ABPM is better in detecting hypertension and monitoring adequacy of treatment in children with CKD. The high prevalence of masked hypertension and its association with LVH supports early echocardiography and ambulatory BP monitoring to evaluate cardiovascular risks in this population.
Keywords
Ambulatory blood pressure monitoring
chronic kidney disease
hypertension
left ventricular hypertrophy
pediatric
Introduction
Hypertension is common in children with chronic kidney disease (CKD) and is a significant risk factor for cardiovascular disease.[123456] It has been shown by previous studies that over 25% of children with CKD had elevated casual blood pressure (CBP), with nearly one-third of these not receiving antihypertensive medications, indicating that hypertension in pediatric CKD may be frequently under-treated or missed altogether.[2]
Ambulatory blood pressure monitoring (ABPM) has been shown to be a more accurate technique than CBP to diagnose hypertension and to stratify cardiovascular risk, especially in patients with CKD stages 3–5.[789] In adults and children with CKD, ABPM has been found to be superior than CBP to diagnose hypertension and to monitor adequacy of treatment.[101112] ABPM also correlates better with end organ damage.[13141516] ABPM uses a wearable, oscillometric BP device that automatically measures and records BPs at fixed intervals (every 20 min when awake and every 30 min to 1 h during sleep) over an entire 24-h period.[17] This allows the assessment of child's overall exposure to elevated BP (BP load) and changes in normal circadian BP pattern. Furthermore, ABPM minimizes the effect of anxiety-induced BP elevations known as white coat hypertension (WCH) as BP is monitored over a longer period in the child's own environment.[1819] ABPM is also able to diagnose masked hypertension, a condition in which CBP in an office setting is normal, but the BP is found to be high at other times during 24 hours.[18] Masked hypertension is not a benign entity and has been shown to predict end organ damage thus necessitating its detection and management.[8]
Although there are some studies that have evaluated the role of ABPM in children with CKD, there is still limited data in this population from India.[27920] This study was, therefore, undertaken to better define the role of ABPM in pediatric patients with CKD. We compared ABPM data in 46 children with CKD stage 3–5 with CBP measurements and correlated our findings with echocardiographic data to study the relationship between ABPM, hypertensive parameters and end organ cardiovascular injury.
Materials and Methods
Study design
This prospective cross-sectional study was conducted at the Pediatric Nephrology division of Christian Medical College and Hospital Vellore, India from October 2011 to November 2012. During this time frame, 400 visits were made to the Pediatric Nephrology OPD by children with CKD, out of which 46 children were included in our study. Children aged 3–18 years with known CKD and estimated glomerular filtration rate (GFR) of < 60 ml/min/1.73 m2 (CKD stage 3–5) were recruited after parental consent. Children on antihypertensive medications were also included. The minimum duration of CKD since diagnosis was 2 months. Estimated GFR was calculated using the Schwartz formula.
Children with renal, other solid organ or bone marrow transplantation, children diagnosed with cancer/HIV during the past 12 months, children who were bedridden, children with genetic syndromes or the presence of any heart disease were excluded.
Methodology
The study was approved by the Institutional Review Board of the hospital prior to initiation. Demographic and medical history, information including age, gender, underlying cause of CKD, a history of hypertension and use of antihypertensive medications were recorded.
Casual blood pressure
Casual blood pressure was obtained by an oscillometric device (OMRON) in the office setting. BP was recorded after 5 min of rest using appropriately sized BP cuffs. The oscillometric devices were calibrated by the quality management cell of our hospital every month. Since the ABPM machine was also based on the oscillometry, we decided to use a sphygmomanometer based on the same principle for measuring the casual BP. If the BP was found to be elevated, repeat BP readings were taken. The mean of three BP readings during the clinic visit were taken. Hypertension was defined as BP ≥95th centile for age, sex and height according to the National High Blood Pressure Education Program (NHBPEP) Fourth Report on the Diagnosis, Evaluation, and Treatment of High BP in Children and Adolescents.[21] If children were on dialysis, the post-dialysis BP was taken since pre-dialysis BP is more likely to be affected by maximal fluid overload and is less reliable. Both CBP and ABPM were measured when the children came to the hospital for checkup. Thus, the timings of CBP and ABPM vary for each child according to the timing of the clinic visit.
Ambulatory blood pressure monitoring
Ambulatory blood pressure was monitored using the Spacelabs 040-1546-00 oscillometric device. The appropriate sized BP cuff as per the Fourth report recommendation was placed on the non-dominant arm of the child and BP readings were recorded for a 24-h period. Subjects were asked to continue all their normal daily activities. BP recordings were measured every 20 min during the daytime and hourly at night. The means of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were determined for 24 h during wake and sleep periods. Using both CBP and ABPM results, BP status was categorized using the following definitions:[1718]
-
Normal BP - Office BP <90th percentile and mean ambulatory SBP or DBP <95th percentile and SBP or DBP load <25%
-
White coat hypertension - Office BP ≥95th percentile and mean ambulatory SBP or DBP <95th percentile and SBP or DBP load <25%
-
Prehypertension - Office BP ≥90th percentile or >120/80 mm Hg and mean ambulatory SBP or DBP <95th percentile and SBP or DBP load <25%
-
Masked hypertension - Office BP <95th percentile and mean ambulatory SBP and DBP >95th percentile, or SBP or DBP load ≥25%
-
Ambulatory hypertension - Office BP >95th percentile and mean ambulatory SBP or DBP >95th percentile or SBP or DBP load 25–50%
-
Severe ambulatory hypertension: Office BP >95th percentile and mean ambulatory SBP or DBP >95th percentile or SBP or DBP load >50%
-
Nocturnal dipping was determined by calculating percent nocturnal drop in mean BP from waking mean values. Abnormal nocturnal dipping was defined as <10% difference between daytime and nighttime BP readings
-
Blood pressure index was calculated as the average BP of the child divided by the 95th centile ambulatory BP for that particular age, height and gender
-
Blood pressure load was calculated as the percentage of BP readings >95th centile.
Normative data for ABPM and the definition of ambulatory hypertension were based on AHA recommendations on ambulatory BP monitoring for children and adolescents.[17]
Echocardiogram
Echocardiogram (ECHO) was done using the Philips IE 33 model with 12-size pediatric probes. The left ventricular mass (LVM) was calculated according to the Devereox formula.[22] Left ventricular hypertrophy (LVH) was defined as the LVM >95th centile for age, sex and height.[23] The LVM index (LVM/Ht) and LVM/Ht2.7 were also calculated.
Statistical analysis
Descriptive statistics were presented as means, standard deviation (SD) and percentages. Continuous variables were analyzed by Student's t-test, whereas Chi-square test or Fisher exact test were used for analysis of categorical variables. Children with LVH were compared with those with normal LVM Index (LVMI) in a univariate analysis. Adjusted odds ratios for LVH were then calculated using a logistic regression model. Only variables with P < 0.05 were included in the multivariable analysis. Results are summarized as odd ratios with 95% confidence intervals. All statistical analyses were performed using SPSS version 15 (SPSS for Windows, Chicago, SPSS Inc).
Results
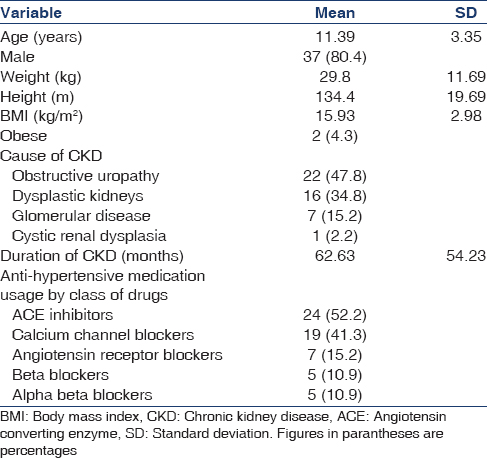
A total of 46 patients were included in the study. While 39.1% children were in Stage 3 and 4 CKD each, 21.7% had Stage 5 CKD. Eleven out of the 46 children were undergoing dialysis (4/11 hemodialysis and 7/11 peritoneal dialysis). The demographic and clinical characteristics of children are shown in Table 1. The major causes of CKD were obstructive uropathy (48%), dysplastic kidneys (35%), glomerular disease (15%) and cystic disease (2%).
Descriptive results of the assessment of hypertension
Of the 46 children, 10 children were found to have elevated casual BP (10/46; 21.7%). ABPM was performed on all children, and 6/46 (13%) were found to have severe ambulatory hypertension [Table 2]. Children with normal casual BP but elevated ambulatory BP were classified as masked hypertension 9/46 (19.6%). In addition 3 (6.5%) children had WCH with elevated casual BP recordings but normal ambulatory BP and normal BP loads. Eight children (17.4%) had unclassified hypertension with both normal casual and ambulatory BP but isolated elevation of BP loads. The significance of isolated elevation of BP load has not yet been defined but according to Samuels et al. and Mitsnefes et al. it can be classified as masked hypertension, especially in a high-risk population like children with CKD.[78] None of the children had ambulatory hypertension (non-severe) or pre-hypertension.

Of the study cohort, 34 children (73.9%) were known hypertensives as detected by CBP and were receiving antihypertensive therapy. At the point of intervention, CBP was elevated in only 8/34 (23.5%), the remaining being normotensive, suggesting adequate BP control. However, using ABPM 4 children were found to have severe ambulatory hypertension (11.7%); 5 had masked hypertension (14.7%); 3 had WCH (8.8%) and 4 had unclassified hypertension (11.7%). Among these children found to have hypertension using ABPM, LVH was detected in 12 children (32.2%).
Among 12 previously normotensive children casual BP was able to detect new-onset hypertension in 2/12 (16.6%), whereas using ABPM 3 children were found to have severe ambulatory hypertension (25%); 4 had masked hypertension (33.3%); 2 had WCH (16.6%) and 3 had unclassified hypertension (25%).
A total of 34 (73.9%) children were receiving anti-hypertensive medications. Angiotensin converting enzyme inhibitors were the commonest medication used (52.2%) followed by calcium channel blockers (41.3%). Only 2 children were taking both ACEI and ARB. Sixteen (34.8%) children were on 1 medication, 9 (19.6%) were on 2 medications, 7 (15.2%) were on 3 medications and 2 (4.3%) were on 4 antihypertensive medications.
The burden of hypertension as assessed by both casual BP readings and ABPM showed an increasing trend with higher stages of CKD [Figure 1]. Across both stages 3 and 5 of CKD, hypertension was missed in children by CBP. In stage 3 CKD, 22.2% (4/18) had elevated CBP and 50% (9/18) had abnormal ABPM readings; in stage 4, 55.5% (10/18) had elevated CBP and 44.4% (8/18) had ambulatory hypertension while in stage 5, 50% (5/10) had elevated CBP and 90% (9/10) had ambulatory hypertension.

- Increasing burden of hypertension with reducing renal function (comparison of casual blood pressure readings versus ambulatory blood pressure monitoring)
Children were found to be chronically exposed to high BP levels as assessed by elevated systolic and diastolic BP index - 14/46 [30.4% Table 2]. Overall 34 children had lack of normal nocturnal dipping of BP. Absence of normal nocturnal BP dipping was seen in 33/46 (71.7%) for systolic BP and 20/46 (43.5%) for diastolic BP. Elevated daytime systolic loads were seen in 36.9%, elevated nighttime systolic load in 56.5%, elevated daytime diastolic loads in 41.3% and elevated nighttime diastolic loads in 58.6% [Table 2].
Left ventricular hypertrophy was defined as the LVM >95th centile for age, sex and height.[23] Using the above definition, 13 children were found to have LVH. Only 6 (46.1%) of these children were identified to have hypertension by CBP, whereas one (7.6%) had normal BP; 3 (23%) had severe ambulatory hypertension, 4 (30.7%) had masked hypertension, 2 (15.3%) had WCH and 3 (23%) had unclassified hypertension as classified by ABPM [Figure 2]. Hence we would have missed many children with LVH if ABPM had not been used. LV hypertrophy was further classified into concentric hypertrophy (elevated LVMI and relative wall thickness >0.41) and eccentric hypertrophy (elevated LVMI and relative wall thickness <0.41).[2425] Eleven children had concentric hypertrophy (suggestive of pressure overload), whereas 4 children had eccentric hypertrophy (suggestive of volume overload).

- Left ventricular hypertrophy was noted in all categories of ABPM in varying proportions
Among the 11 children who were undergoing dialysis, the mean duration of dialysis was 7.5 months (range 0–30 months, Median 3 months). Children on dialysis had a greater LVM (142.6 g) compared to children not requiring dialysis (88.7 g, P = 0.003). There was a statistically significant difference between the LVMI of children with CKD not requiring dialysis (42.5 g/m2.7) and those on dialysis (64 g/m2.7 P = 0.026).
Mean systolic, diastolic and mean arterial pressure as determined by ABPM showed a linear positive correlation with LVM by Echo [Figure 3a–c]. Systolic BP correlated with LVM (regression coefficient = 0.481, P = 0.001); diastolic BP correlated with LVM (regression coefficient = 0.467, P = 0.001) and mean BP correlated with LVM (regression coefficient = 0.478, P = 0.001). Systolic BP by ABPM was also found to correlate linearly with LVM (P = 0.001); LVM/Ht (P = 0.009) and LVM/Ht2.7 (P = 0.375). A greater and significant proportion (P = 0.036) of children on dialysis had LVH (54.4%–6/11) compared to the non-dialysis group (20%–7/35).

- (a) Linear correlation of systolic blood pressure with left ventricular mass in study children (regression coefficient = 0.481, P = 0.001). (b) Linear correlation of diastolic blood pressure with left ventricular mass in study children (regression coefficient = 0.467, P = 0.001). (c) Linear correlation of mean blood pressure with left ventricular mass in study children (regression coefficient = 0.478, P = 0.001)
The three parameters that assess ABPM-BP index, nocturnal dipping and BP load were compared with the echocardiography findings. The number of abnormal ABPM indices correlated with the percentage of patients with LVH (P = 0.046). When ABPM was normal, only 7.7% of subjects had LVH. When 1 ABPM index was abnormal 15.4%; with 2 indices 30.8% and with 3 indices 46.2% had LVH [Figure 4].

- Left ventricular hypertrophy versus ambulatory blood pressure (BP) monitoring indices (nocturnal dipping, BP load and BP index)
Discussion
Cardiovascular risk factors are a leading cause of morbidity and mortality in children with CKD.[26272829] Hypertension is highly prevalent in children with CKD and is an important contributor to adverse cardiovascular profile in children with CKD.[62030] In addition, multiple studies have shown hypertension to be an independent risk factor for CKD progression.[7313233] Early detection of hypertension and adequate control are thus paramount to prevent cardiovascular morbidity, mortality and CKD progression.
In our study, 10/42 (21.7%) had elevated CBP and 9/46 (19.6%) children were found to have masked hypertension. This group included children on anti-hypertensive medications with inadequate BP control as well as children who have been newly diagnosed to have elevated ABPM with normal casual BP readings. This is similar to the high prevalence of masked hypertension found in recent pediatric studies. Mitsnefes et al. reported 38% of children to have masked hypertension by ABPM.[8] Chaudhuri et al. recently reported the presence of masked hypertension in 12% of children with stage 5 CKD on dialysis.[9] The high burden of masked hypertension in our study could be due to the inclusion of both pre-diagnosed children on anti-hypertensive medications as well as children who were newly diagnosed to have hypertension. In India, due to lack of awareness as well as financial constraints children with CKD present to hospital very late in the course of their disease. Thus, the children included in our study would represent only the symptomatic children who are sick and have sought hospital care.
Thirty-four (73.9%) children in our study were known hypertensives receiving anti-hypertensive medications. Using casual BP assessment 8 (23.5%) had poor BP control, whereas using ABPM 16 (47%) had inadequate BP control. This is similar to reports from Mitsnefes et al. who had 54% hypertensives at enrolment with 48% with inadequate BP control despite anti-hypertensive medications.[20]
We found that the prevalence of hypertension increased with worsening stages of CKD and use of CBP alone would have missed the detection of hypertension in more than half the children. This is in keeping with reports from Mitsnefes et al. and Samuels et al.[78]
The ABPM readings for BP were compared with the echocardiography findings. It was noted that the systolic, diastolic and mean BP readings significantly correlated with the LVM (P = 0.001) [Figure 3]. SBP also correlated with LVM/Height (LVMI), but to a lesser extent than with LVM alone (P = 0.009). This is similar to the findings from Sorof et al. who found that LVMI correlated strongest with ambulatory SBP index (r = 0.43, P = 0.008) and also correlated with 24 h SBP (r = 0.34, P = 0.037).[13]
Further, we were interested to find an association between ambulatory hypertension and LVH. We found that of the 13 children with LVH in our study, only 6 were identified to have hypertension by CBP reading, the remaining 7 were identified to have hypertension by ABPM. Hence, more than half of the children who had LVH on ECHO would have been missed if we had gone by casual BP readings only and not done ABPM. Mitsnefes et al. further reported that 34% of children with hypertension and 20% children with masked hypertension had LVH.[8] In our study, 50% of the children with severe ambulatory hypertension; 66.6% of children with WCH; 44.4% of children with masked hypertension and 37.5% of children with unclassified hypertension had LVH.
Ambulatory blood pressure monitoring was assessed using three parameters: BP load, BP index and lack of nocturnal dipping. The correlation between the number of abnormal ABPM indices and the prevalence of LVH was found to be statistically significant (P = 0.046). Similar results can be found in the study by Samuel et al. in which they included children with elevated BP load >25% but normal casual BP and normal 24 h mean BP as having ambulatory hypertension.[7] Their justification is that in children with CKD (high-risk group), elevated BP load >25% alone is a significant risk factor for end organ damage, though this is not included in the American Heart Association's Guidelines for ABPM.[18]
Cardiovascular morbidity increases with advancing kidney disease. Echocardiography with calculation of LVM and LVMass/Ht was a very important indicator of cardiac dysfunction. Our study showed that children on dialysis had greater LVM (142.6 g) compared to those in earlier stages of CKD 3–5 (88.7 g, P = 0.003). Children with dialysis had a significantly higher percentage of LVH (6/11 54.4%) compared to CKD children (7/35 20% P = 0.036). This is lower than the results from Mitsnefeset al. study, which showed that up to 69–82% of children had LVH at initiation of dialysis.[34]
Our study was not without its limitations. There is no normative data on ABPM or LVM for Indian children. Western standards used might not be representative of our population. Thus, further studies are required in India to gather local statistics. Ours was a cross-sectional study, and we were not able to follow the progression of LV hypertrophy in children over time. We were thus not able to demonstrate a regression of LVH after stricter BP control in CKD children unlike that shown by Kupferman et al.[35]
Conclusion
Our observation that ABPM identifies new-onset hypertension and suboptimal hypertension control in a substantial proportion of CKD children reiterates the necessity of ABPM in every child with CKD. The strong correlation between mean systolic, mean diastolic and mean arterial pressure with LVM and LVM index indicates that hypertension is an important contributor to increased LVM in CKD children. The close association that we have demonstrated between indices of hypertension using ABPM and the proportion of children with CKD with LVH clearly shows the superiority of ABPM over casual clinic readings of BP.
Source of Support: Fluid Reserch Grant, Christian Medical College, Vellore
Conflict of Interest: None declared.
References
- Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. 2010;5:2172-9.
- [Google Scholar]
- Blood pressure in children with chronic kidney disease: A report from the chronic kidney disease in children study. Hypertension. 2008;52:631-7.
- [Google Scholar]
- Cardiac geometry in children receiving chronic peritoneal dialysis: Findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clin J Am Soc Nephrol. 2011;6:1926-33.
- [Google Scholar]
- Demographics of blood pressure and hypertension in children on renal replacement therapy in Europe. Kidney Int. 2011;80:1092-8.
- [Google Scholar]
- High prevalence of the metabolic syndrome and associated left ventricular hypertrophy in pediatric renal transplant recipients. Pediatr Transplant. 2010;14:52-60.
- [Google Scholar]
- Hypertension and progression of chronic renal insufficiency in children: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) J Am Soc Nephrol. 2003;14:2618-22.
- [Google Scholar]
- Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012;60:43-50.
- [Google Scholar]
- Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21:137-44.
- [Google Scholar]
- Role of twenty-four-hour ambulatory blood pressure monitoring in children on dialysis. Clin J Am Soc Nephrol. 2011;6:870-6.
- [Google Scholar]
- Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762-8.
- [Google Scholar]
- 2013 Ambulatory blood pressure monitoring recommendations for the diagnosis of adult hypertension, assessment of cardiovascular and other hypertension-associated risk, and attainment of therapeutic goals (summary).Joint recommendations from the International Society for Chronobiology (ISC), American Association of Medical Chronobiology and Chronotherapeutics (AAMCC), Spanish Society of Applied Chronobiology, Chronotherapy, and Vascular Risk (SECAC), Spanish Society of Atherosclerosis (SEA), and Romanian Society of Internal Medicine (RSIM) Clin Investig Arterioscler. 2013;25:74-82.
- [Google Scholar]
- Toward a definition of masked hypertension and white-coat hypertension among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2003-8.
- [Google Scholar]
- Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002;39:903-8.
- [Google Scholar]
- Carotid artery intimal-medial thickness and left ventricular hypertrophy in children with elevated blood pressure. Pediatrics. 2003;111:61-6.
- [Google Scholar]
- Escape Trial Group. Home, clinic, and ambulatory blood pressure monitoring in children with chronic renal failure. Pediatr Res. 2004;55:492-7.
- [Google Scholar]
- Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894-900.
- [Google Scholar]
- Update: Ambulatory blood pressure monitoring in children and adolescents: A scientific statement from the American Heart Association. Hypertension. 2014;63:1116-35.
- [Google Scholar]
- Ambulatory blood pressure monitoring in children and adolescents: Recommendations for standard assessment: A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension. 2008;52:433-51.
- [Google Scholar]
- White-coat hypertension: New insights from recent studies. Hypertension. 2013;62:982-7.
- [Google Scholar]
- Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol. 2012;23:578-85.
- [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555-76.
- [Google Scholar]
- Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613-8.
- [Google Scholar]
- Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709-14.
- [Google Scholar]
- Echocardiography-based left ventricular mass estimation. How should we define hypertrophy? Cardiovasc Ultrasound. 2005;3:17.
- [Google Scholar]
- Impaired left ventricular diastolic function in children with chronic renal failure. Kidney Int. 2004;65:1461-6.
- [Google Scholar]
- Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:191-7.
- [Google Scholar]
- Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Hypertension. 2003;42:1050-65.
- [Google Scholar]
- Cardiovascular complications of pediatric chronic kidney disease. Pediatr Nephrol. 2008;23:27-39.
- [Google Scholar]
- Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int. 2006;70:585-90.
- [Google Scholar]
- Prevalence and correlates of multiple cardiovascular risk factors in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2759-65.
- [Google Scholar]
- Renal microvascular dysfunction, hypertension and CKD progression. Curr Opin Nephrol Hypertens. 2013;22:1-9.
- [Google Scholar]
- Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European study group of nutritional treatment of chronic renal failure in childhood. Lancet. 1997;349:1117-23.
- [Google Scholar]
- The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of diet in renal disease study group. N Engl J Med. 1994;330:877-84.
- [Google Scholar]
- Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol. 2001;16:318-23.
- [Google Scholar]
- BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol. 2014;25:167-74.
- [Google Scholar]







