Translate this page into:
An unusual case of cocoon abdomen in a patient on hemodialysis
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
“Cocoon abdomen” or sclerosing encapsulating peritonitis is a rare cause of intestinal obstruction. It has been described in patients on continuous ambulatory peritoneal dialysis. The exact etiology is unknown, but pathogenesis rests on chronic peritoneal inflammation. No case has been reported so far in patients on hemodialysis. We hereby report a case of cocoon abdomen presenting as refractory ascites with intestinal obstruction in a patient on maintenance hemodialysis.
Keywords
Chronic kidney disease
cocoon abdomen
maintenance hemodialysis
Introduction
“Cocoon abdomen” is a rare cause of intestinal obstruction, first described in young adolescent girls.[1] Initially, cases were either idiopathic or attributed to tuberculosis (TB). Clinical features include abdominal pain, nausea, vomiting, hemorrhagic ascites, repeated bowel obstruction, blood-stained effluent and loss of ultrafiltration capacity in continuous ambulatory peritoneal dialysis (CAPD) patients.[2] CAPD is a common cause of the abdominal cocoon syndrome in patients of chronic kidney disease (CKD); however, no case has been described in patients on maintenance hemodialysis. We hereby report a case of cocoon abdomen presenting as refractory ascites with intestinal obstruction in a patient on maintenance hemodialysis.
Case Report
A 51-year-old female was diagnosed to have essential hypertension since 12 years and CKD since last 4 years. She progressed to end-stage renal disease 2 years back and was initiated on maintenance hemodialysis. For initial 1-year, she was on twice-weekly hemodialysis, which was subsequently increased to thrice weekly. She presented to us with complaints of progressive painless abdominal distension of 8-month duration along with anorexia and weight loss. There was no history of abdominal pain, altered bowel habits, pedal edema, fever, jaundice and no history to suggest hypothyroidism or connective tissue disease. There was no history of diabetes mellitus, jaundice, abdominal surgery and anti-tubercular treatment in past. Her medication included prazosin 10 mg/day, amlodipine 10 mg/day, calcium carbonate 1500 mg/day, twice weekly subcutaneous erythropoietin, oral iron and multivitamin. On examination, she had normal sensorium with blood pressure of 180/120 mm of Hg, pulse rate - 110/min, respiratory rate - 20/min, was afebrile and pale. Her abdomen was symmetrically distended with fluid thrill. There were no visible veins; hernial sites were normal with no cutaneous stigmata of chronic liver disease. Patient had hemoglobin of 7.7 g/dl, total leukocyte count (TLC) of 10400/mm3, with normal differential, platelet count 2.50 lakh/mm3, mean corpuscular volume 90.8 fl, mean corpuscular hemoglobin 30.1 pg/cell, mean corpuscular hemoglobin concentration 33.2 g/dl, normal coagulogram, urea 197 mg/dl, creatinine 9.4 mg/dl, sodium 144 mEq/L, potassium 6.1 mEq/L, chloride 110 mEq/L, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase were 27, 15 and 254 IU/L respectively, bilirubin 0.8 mg/dl, total protein and albumin 6.5 and 2.7 g/dl, calcium 9.7 mg/dl, phosphorous 9.3 mg/dl, and intact parathyroid hormone 346.8 pg/mL. The serology was negative for hepatitis B, hepatitis C and human immunodeficiency virus. On tapping, the ascitic fluid was hemorrhagic and showed TLC of 70/mm3 with 70% polymorphonuclear cells, protein was 3.8 g/dl, sugar 83 mg/dl, with adenosine deaminase of 32 IU/L, and serum ascites albumin gradient of 0.8. The Ziehl–Neelsen stain and Gram-stain were negative, as was the culture for Mycobacterium tuberculosis, and pyogenic organisms. The TB polymerase chain reaction and ascitic fluid for malignant cytology were also negative. Other cancer markers such as cancer antigen (CA) 125, CA 19–9, and carcinoembryonic antigen were also normal. Her electrocardiogram revealed left ventricular hypertrophy with strain pattern, while echocardiography revealed mild mitral regurgitation, left ventricular ejection fraction of 45–50% with no evidence of systolic or diastolic dysfunction. An ultrasound abdomen performed showed gross ascites, bilateral contracted kidneys and cholelithiasis while other abdominal organs were normal. Subsequently, a contrast-enhanced computed tomography (CECT) abdomen was performed which revealed gross ascites with peritoneal enhancement and hyper-densities within fluid, in addition to the finding of contracted kidneys and cholelithiasis [Figure 1]. Suspecting inadequate dialysis, the patient was subjected to daily dialysis. However, there was no improvement in her ascites, and it required repeated therapeutic aspiration. To ascertain the cause of refractory ascites, the patient was subjected to exploratory laparotomy, which showed hemorrhagic ascitic fluid, thickened peritoneum and dense adhesions in-between the bowel loops and bowel loops with the abdominal wall. Keeping the possibility of cocoon abdomen, an intra-operative peritoneal biopsy was taken, which showed hyalinized fibrocollagenous tissue devoid of any lining epithelium with fibrin deposition and inflammatory cell infiltrate. There was no evidence of granulomatous inflammation or malignancy in biopsy specimen [Figure 2]. A diagnosis of cocoon abdomen secondary to uremia was made, and the patient was managed with daily intensive hemodialysis. Over next 1-month, there was gradual improvement in her symptoms along with decrease in ascites.
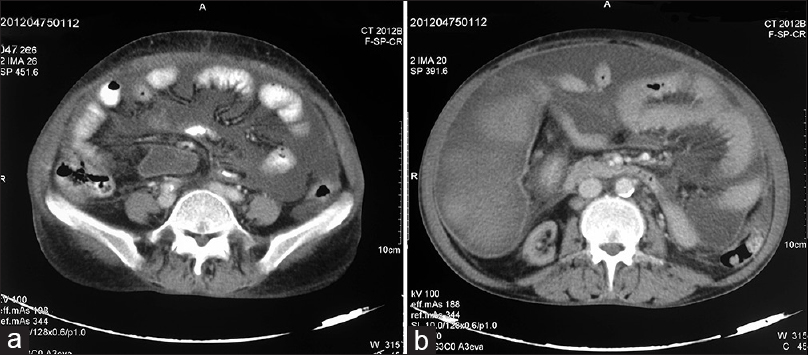
- (a and b) Computed tomography abdomen showing gross ascites with peritoneal enhancement and bowel loop adhesions

- Photomicrograph showing fibrocollagenous tissue with mild inflammatory infiltrate and fibrin deposition (H and E, ×100)
Discussion
Cocoon abdomen, also known variably as sclerosing encapsulating peritonitis (SEP) or sclerosing peritonitis or peritonitis chronica fibrosa incapsulata, is a rare entity characterized by the presence of thick fibrotic peritoneum encasing the bowel loops, leading to intestinal obstruction. Foo et al., were the first to coin the terminology “abdominal cocoon” in context of small intestinal obstruction occurring in young pubertal females with no obvious risk factors.[1] Subsequently, it has been described in chronic peritoneal dialysis,[3] sarcoidosis, systemic lupus erythematosus, practolol therapy, indwelling abdominal catheters, ventriculo-peritoneal and peritoneo-venous shunts, abdominal TB and pelvic inflammatory disease.[4] In patients with CKD, cocoon abdomen is a rare complication in patients on chronic peritoneal dialysis, affecting about 2% of this population and increasing in frequency with the duration of peritoneal dialysis.[5] Some authors have classified this condition as primary and secondary based on whether it is idiopathic (primary) or have any underlying cause (secondary).[6] The exact pathophysiology is not known, but in all conditions associated with cocoon abdomen, there is chronic peritoneal inflammation and subclinical peritonitis[1] culminating into peritoneal fibroneogenesis.[78910] The various imaging technique used in the diagnosis of cocoon abdomen include X-ray abdomen, ultrasonography, computed tomography, magnetic resonance imaging, colonic transit studies and radionuclide studies. The classic barium finding is a concertina-like configuration of dilated small bowel loops in a fixed U-shaped cluster or a “cauliflower sign.”[10] This cauliflower sign is nonspecific and not present always.[9] Among these modalities, CECT provide most characteristic imaging features, with a sensitivity of 100% and a specificity of 94%. The characteristic findings include peritoneal enhancement, peritoneal thickening, peritoneal calcifications, adhesions of bowel loops, signs of bowel obstruction and fluid loculation/septation.[2] The main differential diagnosis is peritoneal encapsulation, which is a developmental anomaly, is usually asymptomatic and is characterized by encapsulation of complete small bowel in a thin accessory membrane.[11] Appropriate management of SEP consists of surgery in the form of membrane dissection and adhesiolysis, along with definitive treatment of underlying disease. Surgery should be conservative to achieve symptomatic relief, as extensive bowel resection is associated with high morbidity.
In our patient, there were no obvious risk factors for cocoon abdomen and investigations for other known etiologies including TB were noncontributory, thus we attributed it to chronic uremic milieu due to inadequate dialysis leading to chronic peritoneal inflammation culminating into cocoon abdomen. We hypothesize that even uremic milieu in itself might be contributing for chronic peritoneal inflammation and eventually to “cocoon abdomen.” This may be supported by the fact that the ascites and symptoms of this patient improved with intensive hemodialysis. Thus, a possibility of cocoon abdomen should be kept in patients of hemodialysis with unexplained refractory ascites with or without symptoms of abdominal obstruction.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Unusual small intestinal obstruction in adolescent girls: The abdominal cocoon. Br J Surg. 1978;65:427-30.
- [Google Scholar]
- Sclerosing encapsulating peritonitis in chronic ambulatory peritoneal dialysis. Clin Radiol. 1990;41:19-23.
- [Google Scholar]
- Case report: Abdominal cocoon associated with tuberculous pelvic inflammatory disease. Br J Radiol. 2002;75:174-6.
- [Google Scholar]
- Encapsulating peritoneal sclerosis: What have we learned? Semin Nephrol. 2011;31:183-98.
- [Google Scholar]
- Abdominal cocoon in a man: Preoperative diagnosis and literature review. J Clin Gastroenterol. 1998;26:148-50.
- [Google Scholar]
- Abdominal cocoon – A cause of intestinal obstruction in a 4 years old girl. Indian Pediatr. 1979;16:1047-8.
- [Google Scholar]
- Abdominal cocoon syndrome (idiopathic sclerosing encapsulating peritonitis): How easy is its diagnosis preoperatively?. A case report. Case Rep Surg 2013 2013:604061.
- [Google Scholar]
- Peritoneal encapsulation and abdominal cocoon. Case reports and a review of the literature. Gastroenterology. 1983;84:1597-601.
- [Google Scholar]
- Abdominal cocoon – A rare cause of intestinal obstruction. Int J Surg Case Rep. 2013;4:955-7.
- [Google Scholar]







