Translate this page into:
Clinical utility of urine neutrophil gelatinase-associated lipocalin measured at admission to predict outcomes in heterogeneous population of critically ill patients
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Urine neutrophil gelatinase-associated lipocalin (uNGAL) is a reliable early biomarker of acute kidney injury (AKI) in a homogeneous patient population. However, its utility in a heterogeneous population of critically ill, in whom the time of onset of renal insult is often unclear, is not clearly established. We evaluated the ability of a single measurement of uNGAL in a heterogeneous adult population, on admission to intensive care unit (ICU), to predict the occurrence of AKI and hospital mortality. One hundred and two consecutive adult patients had uNGAL measured within 8 h of admission to ICU. The demographic and laboratory data were collected at admission. The diagnosis of AKI was based on AKI Network (AKIN) criteria. The primary outcome was the development of AKI, and the secondary outcome was hospital mortality. The mean age was 54 ± 16.4 years and 65% were males. Urine NGAL (ng/ml) was 69 ± 42 in patients with AKI (n = 42) and 30.4 ± 41.7 in those without AKI (P < 0.001). The area under the receiver operating characteristic (ROC) curve for prediction of AKI was 0.79 and for serum creatinine (SCr) was 0.88. The sensitivity and specificity for a cut-off value of uNGAL of 75 ng/ml to predict AKI were 0.5 and 0.85 respectively. uNGAL > 75 ng/ml was a strong (odd ratio = 5.17, 95% confidence interval: 1.39–19.3) and independent predictor of hospital mortality. A single measurement of uNGAL at admission to ICU exhibited good predictive ability for AKI though the sensitivity was low. The predictive ability of uNGAL was inferior to simultaneously measured SCr at admission, hence limited its clinical utility to predict AKI. However, admission uNGAL was a strong, independent predictor of hospital mortality.
Keywords
Acute kidney injury
intensive care unit
urine neutrophil gelatinase-associated lipocalin
Introduction
Acute kidney injury (AKI) is common in critically ill patients. AKI is an independent risk factor for mortality and is associated with increased cost of hospitalization.[1] In clinical practice, the diagnosis of AKI is based on elevated serum creatinine (SCr), which is a late functional biomarker of AKI. The two new diagnostic criteria for AKI namely risk, injury, failure, loss, and end-stage kidney (RIFLE)[2] and AKI network (AKIN)[3] to define AKI also rely heavily on elevated SCr. However, these new criteria to define AKI appear to be flawed, since they take into consideration the functional aspect of AKI, but not the structural injury. Though commonly used in clinical practice, SCr is generally considered an unsatisfactory biomarker for AKI for the following reasons:[4] (1) rise in SCr occurs when glomerular filtration rate (GFR) has already declined by 30–40%, indicating that it is a late marker of AKI, (2) creatinine generation is reduced in septic AKI compared to a normal population, thereby overestimating GFR in sepsis, (3) fluid overload, often seen in critically ill patients, dilutes SCr, thereby underestimating its true concentration in serum, (4) SCr level is affected by age, gender, muscle mass and some medications that affect the generation and excretion of creatinine, and (5) SCr may be increased in functional renal failure such as prerenal azotemia that is often reversible without major adverse consequences.
Interventional studies done so far in human AKI have shown no or equivocal benefit, and the main reason for this failure is attributed to the late clinical diagnosis of AKI based on SCr. There is a tremendous interest in developing a biomarker to diagnose AKI early and reliably, so that interventions to prevent progression of AKI based on this approach could yield positive results. Among the several biomarkers tested in clinical studies, urinary concentration of neutrophil gelatinase-associated lipocalin (NGAL) is the most promising for early detection of AKI.[5] Clinical validation of urine NGAL (uNGAL) in adults is mostly shown in single center studies, performed in homogeneous populations such as post-cardiac surgery,[678] cardio-renal syndrome,[9] post-trauma[10] and post-renal transplantation,[11] wherein the precise timing and the nature of renal injury is known. The utility of uNGAL in predicting AKI is less clear in a heterogeneous population in the intensive care unit (ICU),[8121314] where the precise timing, nature and severity of renal insult is often unknown. Also, the utility of a single measurement of uNGAL at admission to ICU remains unclear due to paucity of data.
We conducted a prospective study in a cohort of a heterogeneous population of critically ill adults to assess the utility of uNGAL measured at admission to predict AKI during the ICU stay and hospital mortality.
Subjects and Methods
We studied prospectively 102 consecutive adult patients admitted to the ICU of a tertiary referral hospital. Approval for the conduct of the study was taken from the hospital ethics committee, and informed consent was obtained from the patient or surrogate decision maker. We excluded patients below 16 years of age, patients with chronic kidney disease with baseline SCr > 1.3 mg/dl prior to admission, organ transplant recipients, patients with known malignancy, pregnant women and patients admitted for observation following cardiac catheterization who were likely to stay in ICU for <48 h.
Preexisting co-morbidities such as diabetes mellitus, hypertension, liver disease and medications were noted. Demographic data such as age, gender, comorbid conditions, clinical setting such as medical or surgical and presence of sepsis were noted. The severity of clinical illness was assessed by Acute Physiology and Chronic Health Evaluation score III[15] and the Sequential Organ Failure Assessment (SOFA) score.[16] The cumulative urine output, initiation of renal replacement therapy, duration of ICU stay, and hospital mortality were recorded.
Definitions
Acute kidney injury was defined based on AKIN criteria.[3] Patients were classified as having either AKI or no AKI and severity of AKI was graded as: (1) Stage-1 corresponding to a 1.5 fold increase in SCr level or an acute rise in SCr of more than 0.3 mg/dl within 48 h (2) Stage-2 corresponding to a 2 fold increase in SCr level and (3) Stage-3, corresponding to a 3 fold increase in SCr or SCr ≥ 4 mg/dl or need for dialysis.
Laboratory measurements
Blood urea, SCr, serum electrolytes and other biochemical parameters of organ function were measured at admission. SCr was measured at admission and thereafter daily. Baseline SCr was defined as the steady state level of creatinine 4 weeks before admission. If not available, the admission value or the lowest SCr during the hospital stay was used as a surrogate baseline. Urine samples were collected within 8 h of admission to ICU and stored at −20°C if delay in analysis was expected. The urine sample thus collected was also analyzed for sodium, urea and creatinine concentration. Fractional excretion of sodium (FENa) and fractional excretion of urea (FEurea) were calculated using standard formulae. Urine NGAL was measured using the BioVendor Human Lipocalin-2/NGAL ELISA, which is a sandwich enzyme immunoassay for the quantitative measurement of human NGAL.
Study outcomes
The primary outcome variable was AKI occurring during ICU stay, and the secondary outcomes were hospital mortality and composite of death and AKI Stage 3.
Statistical analyses
All the continuous data were represented by mean and standard deviation and were analyzed by ANOVA and Student t-test. Categorical data were presented by frequency with percentage and were analyzed using Chi-square test. Receiver operating characteristic (ROC) curves with the area under the curve (AUC) was calculated to predict AKI and composite of death and AKI Stage 3. Multivariate regression analysis was performed to identify the predictors of hospital mortality. Statistical analysis was done using Statistical Package for Social Science (SPSS) version 17.1 (Chicago, IL, USA). A p-value <0.05 was considered as significant.
Results
The mean patient age was 54 ± 16.4 years and 65% were male. The incidence and evolution of AKI and their outcome in our study population are shown in Figure 1. The baseline demographic and laboratory characteristics in patients with and without AKI are shown in Table 1. The comparison of hospital outcomes between AKI and non-AKI patients is shown in Table 2. Table 3 shows the baseline laboratory characteristics and outcome in different classes of AKI. The uNGAL concentration (ng/ml) was significantly higher in patients who died compared to those who survived (84.4 ± 52.7 vs. 41.7 ± 43, P = 0.003). Urine NGAL level was similar in patients with sepsis (62 ± 51 ng/ml) and without sepsis (42.5 ± 43.8 ng/ml) (P = 0.095). In a subgroup of patients who had no AKI at admission, uNGAL measured at admission was significantly higher in those who developed AKI later during the course of ICU stay (n = 9), compared to those never developed AKI (72 ± 41 vs. 30.43 ± 41.6, P = 0.007). The sensitivity, specificity, positive and negative predictive values for uNGAL (ng/ml) cut-off value of 50, 100 and 150 to predict AKI are shown in Table 4.
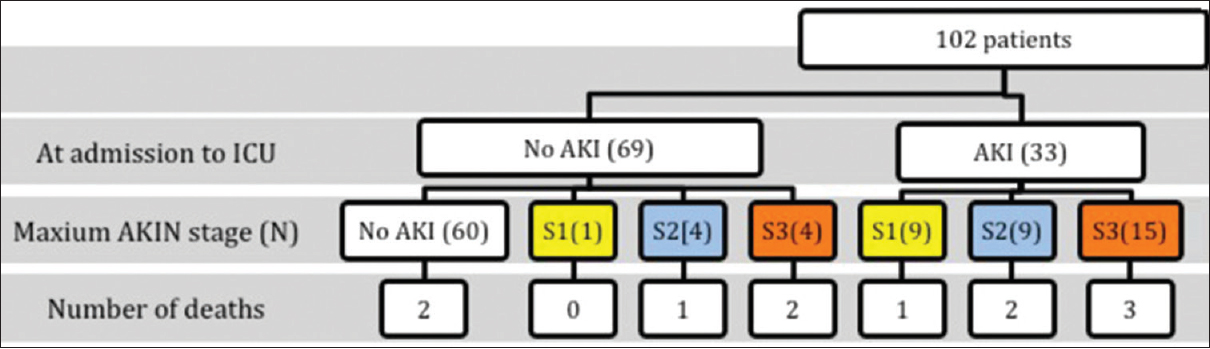
- The incidence, pattern and evolution of acute kidney injury (AKI) in the study population. (S1: Stage 1 AKI, S2: Stage 2 AKI, S3: Stage 3 AKI)




The result of logistic regression analysis to identify the independent risk factors for death is shown in Table 5. Figure 2 shows the AUC for ROC for uNGAL, SCr, FENa and FEurea to predict the development of AKI. Figure 3 shows AUC of ROC for uNGAL and SCr to predict the composite outcome of death and AKI Stage 3 (severe AKI). The odds ratio of hospital mortality were 3.94, (95% confidence interval (CI): 1.07–14.5) for a cut-off value of 50 ng/ml, 5.17 (95% CI: 1.39–19.3) for a cut-off value of 75 ng/ml and 8.2 (95% CI: 2.1–31.4) for a cutoff value of 100 ng/ml for uNGAL.


- Receiver operating characteristics (ROC) curves for prediction of acute kidney injury. (Area under the curve of ROC for urine neutrophil gelatinase-associated lipocalin = 0.79, serum creatinine = 0.88, fractional excretion of sodium = 0.56 and fractional excretion of urea = 0.35)

- Receiver operating characteristics (ROC) curves for prediction of composite of death and acute kidney injury Stage-3. (Area under the curve of ROC for urine neutrophil gelatinase-associated lipocalin = 0.75, serum creatinine = 0.73)
Discussion
Urine NGAL has been proposed as a sensitive and specific marker of AKI in critically ill patients and is shown to predict AKI even before a clinical diagnosis of AKI is made based on RIFLE or AKIN criteria.[612] A single measurement of uNGAL at ICU admission to predict the development of AKI constitutes an appealing approach for the clinician; however, this has not been explored sufficiently in heterogeneous populations of critically ill patients. We studied the predictive ability of uNGAL measured at the time of admission to ICU and compared with other markers of AKI such as FENa and FEurea. Our findings are summarized below.
First, though the incidence of AKI (41%) in our study was similar to that observed in the developed world, the pattern of AKI differed from them. Among the patients who had AKI, 78.5% (33/42) already had established AKI at the time of admission to the ICU and only 21.5% (9/42) developed AKI later during the hospital stay [Figure 1], indicating that admission to ICU was significantly late in the course of their critical illness in our study population. This is in contrast to AKI observed across ICUs in developed countries as reported by Hoste et al., wherein AKI was established at admission in 33% and 67% of AKI was observed after admission.[17] In our study, 10 patients (24% of AKI patients) did not progress beyond the AKIN Stage-1, indicating that some patients in this group probably had functional renal failure (transient AKI or prerenal azotemia) without any significant renal tubular injury. The incidence of transient AKI was slightly higher (24% of all AKI) in our study than the 18% observed by Hoste et al.[17] In such situations, SCr may be elevated due to decreased GFR, but without elevation in uNGAL due to lack of significant tubular injury.[13]
Second, the key finding of our study was that a single measurement of uNGAL at the time of admission to ICU exhibited good ability to predict AKI (AUC of ROC = 0.79) but was inferior to simultaneously measured SCr (AUC of ROC = 0.88). Our results are in contrast to the study by Nickolas et al., who reported excellent prediction of AKI by uNGAL (AUC of ROC = 0.95) with high sensitivity (0.9) and specificity (0.99) of a single measure of uNGAL done in the emergency room.[13] However, not all patients in this study needed ICU admission and hence may not represent a similar study population to ours. Our results concur with those reported by Siew et al., and Bagshaw et al., who showed moderate predictive ability of uNGAL in a heterogeneous adult population in ICU.[1214] Siew et al., in a large study of 451 patients reported AUC of ROC = 0.71 for uNGAL in predicting AKI and concluded that its additional contribution to conventional clinical risk predictors appeared limited.[12] Bagshaw et al., in a study of 83 patients admitted to ICU reported a modest predictive ability of uNGAL (AUC of ROC = 0.7).[14] The reason for an inferior predictive ability of uNGAL compared to SCr in our study is due to following reasons: (1) 24% of AKI patients had transient AKI (AKIN Stage 1) in whom SCr may be elevated but not uNGAL, thereby blunting the ability of uNGAL to predict AKI, and (2) AKI already existed in most (78.5%) who were diagnosed to have AKI during their ICU stay, the diagnosis of which was based on the criteria of elevated SCr concentration. Variability in the prediction of AKI by uNGAL could be attributed to the type, timing and nature of renal insult peculiar to our patient population. It is not uncommon to see critically ill patients being admitted to tertiary referral centers late in the course of their illness in India, wherein AKI is already established at the time of admission. This could be due to late referral by primary and secondary care physicians to a tertiary referral center. Also, the high cost of ICU care is a major deterrent for self-paying patients, resulting in delayed admission to ICU. Our results indicate that the utility of uNGAL vastly depends on the type of patient population in the ICU. We feel that its utility is markedly limited in ICU where referral is late, wherein AKI has already established at admission in most of those who ultimately suffer from AKI. Hence each ICU, especially in the developing world, should explore the utility and assess the cost effectiveness of a policy of routinely measuring uNGAL on admission to ICU. However, admission uNGAL was significantly higher in those who developed AKI after admission than those who never developed AKI, indicating that uNGAL is indeed an early marker of AKI. Hence, despite limitation in a clinical setting as ours, uNGAL can still be a useful early prognostic marker of AKI.
Third, uNGAL measurement at admission lacked sensitivity but had a good specificity. A cut-off value of 50 ng/ml showed the best sensitivity and a cut-off value of 100 ng/ml showed the best specificity [Table 4]. Based on the results of our study, we feel that any value of more than 75 ng/ml should be interpreted as an indicator of renal tubular injury, which had better overall sensitivity and specificity. Currently, standard reference values of uNGAL to diagnose AKI are not available.[18] Normally, uNGAL is below 25 ng/mL and levels above 150 ng/mL are strongly suggestive of AKI.[18] Our results are in concordance with these findings.
Fourth, a single measure of uNGAL at admission was a strong and independent predictor of hospital mortality. In addition, uNGAL was a strong and better predictor of worse outcomes in AKI, a composite of death and severe AKI (AKI Stage 3), than SCr (AUC of ROC: 0.75 vs. 0.73). The risk of hospital mortality increased with increasing levels of admission uNGAL. The prediction of mortality by uNGAL was independent of SOFA score and development of AKI, the other significant risk factors of mortality in our population. This finding is in agreement with recent reports by Siew et al.,[12] Bagshaw et al.,[14] and Yang et al., who reported that uNGAL was an independent predictor of adverse outcome in patients with AKI.[19] We feel that uNGAL has a greater potential as a marker of adverse outcome such as severe AKI and death in critically ill patients. This has major implications in terms of clinical management of AKI whereby patients who are likely to have AKI as well as a worse outcome may accurately be identified by a single measure of uNGAL at ICU admission. In addition, we hypothesize that aggressive hydration, avoidance of nephrotoxins and titration of vasopressors in patients with high admission uNGAL may possibly alter their course and improve outcomes.
Fifth, uNGAL concentration was superior to uNGAL corrected for creatinine excretion (uNGAL to creatinine ratio) in predicting AKI. Delanaye et al., suggested that uNGAL should be corrected for urinary creatinine excretion to reduce the variability in the uNGAL value.[20] However, their conclusions were based on measurements in healthy individuals and not on critically ill patients. Our results indicate that correction for urinary creatinine excretion to express NGAL in urine does not improve its ability to predict AKI and hence may not be necessary.
Sixth, FENa and FEurea at admission to ICU did not predict the development of AKI in our patient population. Analysis of fractional excretion studies showed that FENa as well as FEurea were lower in AKI Stage 1 compared to AKI Stages 2 and 3, and hence could be useful in differentiating transient AKI from progressive AKI. However, their discriminative power was not strong enough to be of value in routine clinical application. A similar finding of FENa not being reliable enough to clinically differentiate transient AKI from persistent AKI was reported by Pons et al., and Bagshaw et al.[2122]
There are several strengths to our study. First, ours is one of the few studies that assessed the utility of a single measurement of uNGAL at admission to ICU in a large heterogeneous group of ICU patients. Second, ours is the first report from developing countries that assesses the utility of uNGAL, where the patient population may not be similar to that of developed countries.
There are several limitations to our study. First, the study was done from a single center. Though we feel that our results generally apply to ICUs in developing countries, our finding needs to be confirmed from other such centers. Second, we excluded patients with preexisting renal impairment from our study, and our results may not apply to patients with acute on chronic renal failure in the ICU.
Conclusions
A single measurement of uNGAL at the time admission to ICU exhibited good predictive ability for AKI though the sensitivity was low. The predictive ability of uNGAL was inferior to simultaneously measured SCr measured at admission, hence limited its clinical utility to predict AKI. However, admission uNGAL was a strong and an independent predictor of hospital mortality.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-70.
- [Google Scholar]
- Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-12.
- [Google Scholar]
- Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31.
- [Google Scholar]
- Marking renal injury: Can we move beyond serum creatinine? Transl Res. 2012;159:277-89.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for early acute kidney injury. Crit Care Clin. 2011;27:379-89.
- [Google Scholar]
- Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485-91.
- [Google Scholar]
- Rapid detection of acute kidney injury by plasma and urinary neutrophil gelatinase-associated lipocalin after cardiopulmonary bypass. J Cardiovasc Pharmacol. 2009;53:261-6.
- [Google Scholar]
- Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012-24.
- [Google Scholar]
- The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: A multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752-61.
- [Google Scholar]
- Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47:79-82.
- [Google Scholar]
- Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006;6:1639-45.
- [Google Scholar]
- Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20:1823-32.
- [Google Scholar]
- Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810-9.
- [Google Scholar]
- Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36:452-61.
- [Google Scholar]
- The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619-36.
- [Google Scholar]
- The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-10.
- [Google Scholar]
- RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73.
- [Google Scholar]
- Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: A review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50:1505-17.
- [Google Scholar]
- Urine neutrophil gelatinase-associated lipocalin: An independent predictor of adverse outcomes in acute kidney injury. Am J Nephrol. 2010;31:501-9.
- [Google Scholar]
- Urinary NGAL measurement: Biological variation and ratio to creatinine. Clin Chim Acta. 2011;412:390.
- [Google Scholar]
- Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: Multicenter cohort study. Crit Care. 2013;17:R56.
- [Google Scholar]
- Urine biochemistry in septic and non-septic acute kidney injury: A prospective observational study. J Crit Care. 2013;28:371-8.
- [Google Scholar]







