Translate this page into:
Nonspecific positivity on the Luminex crossmatch assay for anti-human leukocyte antigen antibodies due to antibodies directed against the antibody coated beads
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Two cases are described of previously unreported false positivity on the Luminex crossmatch assay due to non HLA specific antibodies directed against the beads. In both cases the Luminex crossmatch indicated the presence of donor specific antibodies to class II HLA antigens, which was not substantiated by the clinical scenario or other assays. We could demonstrate the non specificity of these antibodies through using the same assay in a modified form where beads were unexposed to cell lysate and therefore did not carry HLA antigens at all. These cases further serve to emphasize the absolute necessity of correlating positive results with the priming history, and confirming their relevance using other platforms.
Keywords
Crossmatch
false positivity
Luminex
nonhuman leukocyte antigen antibody
transplant
Introduction
Solid phase assays have revolutionized the approach to pretransplant screening by providing unparalleled sensitivity and specificity in the detection of antihuman leukocyte antigen (HLA) antibodies. Yet, instances of false positive results on these platforms have been reported. These have been mainly attributed to the formation and presentation of neo-epitopes during antigen processing and attachment.[123] Such “false positivity” has more commonly been described with assays that use precoated antigenic targets such as Enzyme-linked immuno-sorbant assays (ELISA) or the Luminex single antigen assay. False positivity with the Luminex crossmatch (LumXm) that uses donor lysate as an antigenic source has been less frequently reported, possibly because this assay is less extensively used than the single antigen assay. We report two such cases encountered in our laboratory when using the LumXm.
Methods
Undiluted patient's serum was used for all the assays other than complement dependent cytotoxicity (CDC).
Lymphocyte separation for the crossmatches
Blood was collected in acid citrate dextrose anticoagulant. Lymphocytes were separated by density gradient centrifugation using Ficoll Hypaque and suspended in phosphate buffered saline.
Luminex crossmatch
Luminex crossmatch (Lifecodes Donor Specific Antibody, Tepnel Lifecodes, Connecticut, USA). The LumXm incorporates a blend of Luminex beads, each with a unique fluorescent signature. Two among the beads are coated with monoclonal antibodies specific for class 1 or class 2 HLA. These beads will capture the respective donor HLA antigens when exposed to them in a lysate preparation. After antigen capture, these beads provide an HLA target for detection of donor-specific antibodies [Figure 1].
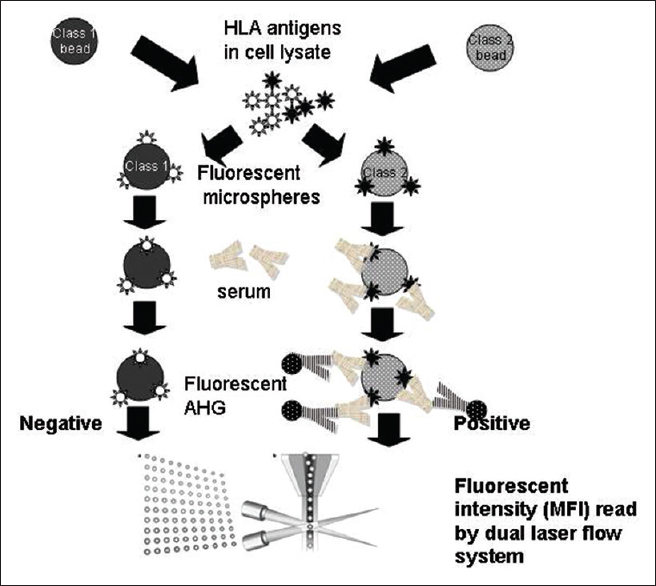
- The Luminex crossmatch protocol: (1) Test beads coated with antibodies that capture donor human leukocyte antigen (HLA) antigens are incubated with the lysate prepared from donor lymphocytes (2) the class 1 and class 2 beads are coated with the respective antigens (3) subsequently the patient's serum is added and at this point of time donor specific anti HLA antibodies if present will attach to the corresponding antigens (4) this reaction is tagged using fluorescent antihuman globulin (5) the reaction is read on a dual laser flow system and quantified in terms of mean fluorescent intensity
Procedure
The LumXm was performed as per the manufacturer's instructions. To prepare donor lysate, 10 μL of the lymphocyte pellet containing approximately 30 × 106 lymphocytes was mixed with 100 μL of lysis buffer. After 10 min, the vial was spun at 2500 RPM for 3 min. The supernatant that constitutes the lysate was aliquoted into tubes and stored at −80°C until use.
For the assay, 8 μL of thawed lysate was incubated with 5 μL of capture beads for 30 min at room temperature in the dark with mixing at 10 min intervals. A volume of 42 μL of kit wash buffer was added, and 55 μL of this mixture added to a well of a prewet filter plate, following which three washes with wash buffer using a vacuum pump were performed. A volume of 38 μL of specimen diluent and 12 μL of patient's serum were added, and the tray was incubated on a shaker for half an hour in the dark. Following three washes, 50 μL of IgG phycoerythrin conjugate in 1:10 dilution was added. Following one more 30 min incubation in the dark and addition of 150 μL of wash buffer, the reading was taken on the Luminex 100 analyzer.
For the auto crossmatch, lysate was prepared from the patient's own lymphocytes.
For performing the procedure with native beads, incubation of the beads with donor lysate was omitted. Other steps remained the same. Mean fluorescent intensity (MFI) readings in the test well above 1000 were taken as positive.
Quality control
All controls were verified to be within set limits prior to validating the test. In the bead mixture, there are three control beads that measure background fluorescence. The test is considered invalid if any of these showed MFI values exceeding 300.
A positive control bead coated with IgG is also included to test for binding of anti-human globulin fluorescein isothiocyanate conjugate. A minimum MFI of 10,000 is required for test validation.
In parallel with the test well, another well called the lysate control is processed to test for attachment of donor HLA antigens to the bead. In this well, after the beads (the same as used in the test) are incubated with lysate, a lysate control reagent containing biotynilated monoclonal antibodies to class 1 and 2 HLA antigens is added. Subsequently, a conjugate of streptavidin phycoerythrin (SAPE) is added, and the reaction read. An MFI of at least 10,000 for class 1 and 7000 for class 2 is required for validation. The bead mixture also contains a biotin coated bead to assure the performance of SAPE and this bead in the lysate control well is to show a minimum MFI of 10,000 for validation. Moreover, the positive control bead in the lysate control well, and the biotin coated bead in the test well need to be negative.
Complement dependent cytotoxicity crossmatch was performed using neat, 1:2 and 1:4 dilutions of plain and dithiothreitol treated serum of the patient, and lymphocytes (B and T cells were not separated) of the donor (or patient for auto crossmatch). Standard (half an hour prior to, and 1 h after, addition of complement) and extended (double of standard) incubation timings at room temperature were used. Appropriate controls (positive, negative and diluent) were used.
Single antigen assay (Tepnel, USA) was performed in accordance with the manufacturer's instructions with incorporated controls.
Enzyme-linked immuno-sorbant assay mixed antigen assay (LAT M, One Lambda, USA) was performed according to the manufacturer's instructions with incorporated controls and the reaction was interpreted as per the manufacturer's calculations.
Case 1
A 44-year-old female was worked up for renal transplant with her sister, who was HLA identical for class 1 (HLA A and B) and 2 (HLA DRB1 and DQ) loci, as the donor. The patient had no history of transfusions or prior transplants but had been pregnant twice. The CDC crossmatches were negative for auto and donor recipient antibodies. An ELISA mixed antigen screen for the patient was also negative for class 1 and 2 anti HLA antibodies. However, the LumXm, while negative (MFI 159) for class 1, was strongly positive (MFI 6972) for class 2. A repeat of the assay using the same serum sample showed MFI values of 107 and 5211.5 for class 1 and 2 respectively. The patient and donor being HLA identical siblings, a possible auto antibody was suspected. Therefore, an auto crossmatch on Luminex was performed using lysate from the patient. This showed MFIs of 134 and 7270 for class 1 and 2 respectively suggesting auto antibodies. However, the absence of their detection on CDC and ELISA despite a strong positivity on the Luminex was intriguing. We hypothesized that the antibodies being detected were directed at an epitope uniquely present on the Luminex beads rather than HLA antigens. To confirm this, we performed the DSA without incubating beads with lysate that is, the beads used here were not coated with HLA antigens. The MFIs obtained were 317 for class 1 and 8859 for class 2 which supported our hypothesis. To confirm these results, we repeated the assay without lysate with a new lot of reagents which showed MFIs of 762 and 16,332 for class 1 and 2 respectively.
The patient was subsequently transplanted uneventfully. Luminex single antigen assay was performed on this serum later when it became available at our center and was found to be negative.
Case 2
A 51-year-old male with no history of transfusions or prior transplants was worked up for transplant with his wife. They were mismatched for 8 of 8 typed antigens (A, B, DRB1 and DQB1 loci). The results of CDC crossmatches and LumXmes, which were performed serially, are shown in Table 1.

The CDC results pointed at a fluctuating IgM autoantibody. However, the LumXm suggested that there was IgG antibody to class 2 in the background, at levels that may not be detected by CDC. Hence, the serial follow-up using Luminex.
Here, again the absence of a priming history, and the autoantibody detected on CDC prompted us to examine the possibility of the antibody being detected on LumXm being an auto antibody rather than an alloantibody. Auto crossmatches on the Luminex platform using the serum of September 26, 2012 and January 18, 2013 showed borderline values for class 2 (MFIs of 982 and 642 respectively).
A Luminex single antigen assay (Tepnel, USA) was performed on the serum of July 02, 2013 and was negative for both class 1 and class 2 antibodies. In the light of the previous case, we performed the LumXm using the serum of September 02, 2013 omitting the incubation of beads with donor lysate, and found the class 2 MFI to be strongly positive that is, 4131.5. This result as well as the negative result on the single antigen assay led us to conclude that the IgG antibody detected on the LumXm assay was not directed at HLA antigens at all but at an epitope uniquely present on the class 2 LumXm beads, possibly on the capture antibodies coating the bead.
Discussion
The LumXm unites the advantages of cell based assays and solid phase assays. It detects donor specific antibodies using antigens derived from the actual donor, while offering high sensitivity, as well as specificity toward the detection of anti HLA antibodies. Other solid phase assays including the Luminex single antigen assay use preattached antigens derived by recombinant technology or from donor lymphocytes.
False positivity has been reported on the Luminex single antigen assay and is attributed to denatured antigenic epitopes produced during processing. These are not present on native lymphocytes as evidenced by negative flow cytometric crossmatches.[123] Case reports suggest that antibodies to the denatured epitopes do not affect the graft.[23]
Such positivity has not been reported in the LumXm assay that uses native antigens extracted from the lymphocyte membrane after membrane lysis using detergent.
The native bead in the crossmatch format is coated with antibodies against a nonvariable portion of the HLA antigen enabling its attachment. Therefore, the interfering antibodies detected in our cases may be directed either against these antibodies or against the bead. While the phenomenon was observed with various lots of the crossmatch assay, thereby excluding a lot related idiosyncrasy, the fact that this was not observed on the Luminex single antigen bead assay suggests that the reactivity is directed against the coating antibody. We also processed three known HLA antibody negative sera (from nonsensitized males with negative antibody screens) and one highly positive serum without lysate. The MFIs for class 2 ranged from 491 to 996 for the negative sera and were 1078 with the positive serum. These values though ranging from negative to weakly positive did not compare with the extremely high reactivity to the beads noted in the patients described above.
Though we could not define the epitope involved, the presence in these patients of human antibodies (such as human anti mouse antibody) against animal antibodies was considered as a possible cause. Such antibodies have been known to interfere with assays that use animal antibodies, causing false positivity by bridging capture and signal antibodies. The single antigen assay that does not use capture antibodies would not be affected.[4]
The observation of such reactivity exclusively against the bead meant for class 2 detection was also intriguing. The antibodies coating class 1 and class 2 beads obviously must carry some structural differences at their antigen binding site that enable them to specifically pick up the class of antigens intended. It is uncertain whether there are other differences such as source or processing methods that could also account for this.
The value of the LumXm in actually predicting graft survival has often been questioned, particularly with respect to positivity for class 2 antigens.[5] In studies where it was compared with flow cytometry and Luminex single antigen assays, it was found to show more discrepant results, particularly for class 2. The failure of the assay to detect antibodies to DQ and DP is a known limitation. However, false positivity that occurred in some patients in these studies is not further elaborated.[67]
Though only two cases are mentioned here, it is hoped that awareness of this phenomenon will aid its further recognition. Moreover, the impact on an individual case must not be underestimated. The strong MFI in cases such as the first would prevent patients from being cleared for transplant if considered in isolation. This report further serves to emphasize the absolute necessity of correlating positive results on antibody screening assays with the priming history and confirming their relevance using additional platforms.
Source of Support: Internal
Conflict of Interest: None declared.
References
- Antibodies against denatured HLA class II molecules detected in luminex-single antigen assay. Hum Immunol. 2013;74:1300-3.
- [Google Scholar]
- Donor-specific antibody against denatured HLA-A1: Clinically nonsignificant? Hum Immunol. 2011;72:492-8.
- [Google Scholar]
- Molecules detected in luminex single-antigen bead assays. Transpl Immunol. 2011;25:77-81.
- [Google Scholar]
- Clinical relevance of Luminex donor-specific crossmatches: Data from 165 renal transplants. Tissue Antigens. 2009;74:205-12.
- [Google Scholar]
- Comparative performance evaluation of a donor-specific bead-based crossmatch technique for the detection of donor-specific anti-HLA antibodies in heart transplantation. Transplant Proc. 2013;45:2005-8.
- [Google Scholar]







