Translate this page into:
Epstein-Barr virus-positive multiple myeloma following an ABO incompatible second renal transplantation
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
ABO incompatible kidney transplant recipients receive higher dose of immunosuppression. Previous data indicate that the incidence of malignancy is not higher in these patients. Compared to the general population, renal transplant recipients are at 4.4-fold higher risk of developing myeloma. We describe a case of posttransplant multiple myeloma in an ABO incompatible renal transplant recipient of a second graft.
Keywords
ABO incompatible
Epstein-Barr virus
multiple myeloma
posttransplant lymphoproliferative disease
renal transplantation
Introduction
Posttransplant lymphoproliferative disorders represent a group of heterogeneous neoplasms. Epstein-Barr virus accounts for the cause of majority of these neoplasms. Adults are usually immune after the primary infection during childhood. ABO incompatible renal transplantation represent a state of heightened immunosuppression. 2nd renal transplant and immunosuppression used for desensitization in ABO incompatible renal transplant could be the reason for development of a hematological malignancy in our case.
Case Report
A 56-year-old patient underwent spousal renal transplantation in September 2011. No induction was given. On postoperative day 2, he had graft thrombosis due to a surgical cause, and graft nephrectomy was done.
Patient's elder brother with A1 positive blood group was found suitable for renal transplantation. The following desensitization protocol was used in this patient for second renal transplantation [Figure 1]. One dose of rituximab 375 mg/m2 was given on day -30. Twenty-six days after rituximab injection, desensitization was carried out with three sessions of anti A antibody removal by an antigen specific immunoadsorption using Glycosorb A@ column. Immunosuppression with tacrolimus 0.75 mg/kg/day, mycophenolate mofetil 1000 mg/day, and prednisolone 10 mg/day was started on day -30. We did not measure tacrolimus levels prior to transplantation. Induction with basiliximab 20 mg was given on day 0 and day 4 of the surgery. Anti A antibody titer was 1:2 prior to transplantation (not shown in graph).
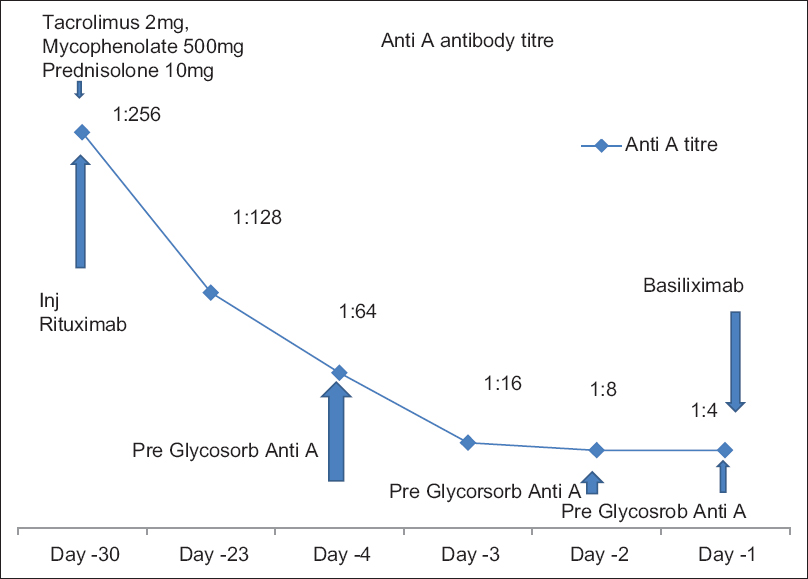
- Desensitization protocol used
Renal transplantation was performed on November 22, 2011. HLA was a 3/6 match. Cross match by complement dependent cytotoxicity was 10%. He was discharged on postoperative day 5 with triple immunosuppresion of prednisolone 30 mg once daily, tacrolimus 0.1 mg/kg/day (3 mg twice daily), and mycophenolate mofetil 500 mg twice daily. Tacrolimus level was maintained between 6 and 10 ng/ml.
One year after the transplantation, he developed left frontal bone osteomyelitis due to methicillin resistant Staphylococcal aureus which was treated with linezolid for 21 days.
Two years later, he developed low back ache. Magnetic resonance imaging of the thoracolumbar spine was normal. However, he developed graft dysfunction with a creatinine of 2.3 mgdl with proteinuria and microscopic hematuria. Hemoglobin was 10 gdl, total count was 5100 cells with a normal differential count and normal platelet count. Anti A antibody titer was 1:2. Serum globulin was 7.6 gdl with serum total protein of 11.0 gdl. There was no hypercalcemia. Transplant kidney biopsy was suggestive of mild interstitial fibrosis and tubular atrophy with no features of cast nephropathy, light chain deposition disease or rejection. Immunofluorescence study revealed no deposits. Serum immunofixation electrophoresis was suggestive of kappa light chain monoclonal gammopathy. Free kappa levels were 12,660 mg/L and kappa lambda ratio was 25.59:1. Bone marrow aspiration showed 59% plasma cells and bone marrow biopsy revealed nodular plasma cell aggregates. Immunohistochemistry was suggestive of CD 138 plasma cells. Epstein-Bar virus (EBV) DNA was positive with titers of 12,567 copies/ml.
He was started on triple drug regimen of bortezomib 2 mg on day 1, 8, 14, and 28 of the month; dexamethasone 40 mg on day 1, 8, 14, and 28 of the month; and oral cyclophosphamide 500 mg on day 1, 8, 14, and 28 of the month.
Patient was maintained on steroids as the sole immunosuppressant on other days. After 6 months of treatment, patient was well with no complaints. There was a marked reduction in total protein to 6.6 gdl, globulin levels to 2.1 gdl, and normalization of the kappa lambda ratio. Serum creatinine was 1.2 mgdl and there was no proteinuria or microscopic hematuria.
Discussion
The risk of malignancy after ABO incompatible renal transplantation is same as that of ABO compatible renal transplantation. Yamamoto et al. analyzed the risk of malignancy in these patients. ABO incompatible kidney transplant recipients were older than ABO compatible kidney transplant recipients and splenectomy was performed in all the ABO incompatible transplant recipients. In this study despite increased age and splenectomy, there was no difference in the incidence of malignancy between ABO incompatible kidney transplant recipients and ABO compatible kidney transplant recipients (4.8% and 4.2%).[1] Hall et al. showed that 7 of 318 ABO incompatible kidney transplant recipients experienced posttransplant cancer.[2]
Post-transplant lymphoproliferative disorders (PTLD) are a group of heterogeneous neoplasms that are associated with EBV infection. Among the hematological malignancies occurring following transplantation, lymphomas are the most common followed by leukemia and multiple myeloma. Factors predisposing to this increased risk of malignancy include impaired immunity due to use of immunosuppressive drugs and primary infection or recrudescence of viral infections with oncogenic potential.[3]
Most cases of PTLDs occur in the first post transplant year. The more intense the immunosuppression used the greater the risk of lymphomas and they occur early. Non-Hodgkin lymphoma accounts for 93% of the cases.[4] Post renal transplant multiple myeloma is a very rare neoplasm accounting for <4% of all PTLDs.[5] Other case series describe the incidence of the disease to be <1%.[6] However, compared to the general population, renal transplant recipients are at 4.4-fold higher risk of developing multiple myeloma and 10-year survival for posttransplant myeloma was 26% lower as compared to nonHodgkin's lymphoma.[7] The Indian scenario has been highlighted by Sakhuja et al. where 40 malignancies were diagnosed in 36 patients after transplantation. The incidence of posttransplant lymphoproliferative disease was 1.45% and only 7 patients had multiple myeloma accounting it to be 24.13% of all lymphomas.[8]
This case report illustrates a rare case in which posttransplant lymphoma presented as EBV-positive multiple myeloma after receiving aggressive immunosuppression for ABO incompatible kidney transplantation. We, however, did not measure Epstein-Barr serological status of both donor and recipient before transplantation as it is not a routine protocol.
EBV infection produces self-limiting illness in young adults and persists as latent infection in B-cells. In vitro, EBV can transform B-lymphocytes into immortalized lymphoblastoid cell lines.[9] EBV-specific cytotoxic T-lymphocyte responses are impaired in patients who are treated with immunosuppressants and this in turn can lead to lymphoproliferative disorders.[10] Role of EBV infection in the genesis of multiple myeloma after transplantation has not been well elucidated. Given the later occurrence of multiple myeloma after transplantation as compared to other posttransplant lymphoproliferative disease, implication of an oncogenic viral infection following institution of aggressive immunosuppression regimens in the early posttransplant period remains highly speculative, despite the demonstration of EBV infection in a patient with multiple myeloma.[11]
The risk factors for the multiple myeloma were different in various studies. In a study from the French registry, Caillard et al. reported that posttransplantation myeloma was associated with older age, transplantation from deceased donor, and antithymocyte globulin treatment.[12] Cyclosporine and OKT3 induction regimen was used in a patient who developed post transplantation multiple myeloma after 3 years of transplantation.
In a study, plasmacytoma/multiple myeloma was described only in 5 out of 78 patients with PTLD.[13] About 75% of the multiple myeloma EBV-positive. EBV-negative post transplant myeloma has also be been reported. Association of multiple myeloma with hepatitis C virus infection has also been described. Multiple myeloma has been described as early as 2 months after transplantation, keeping aside donor transmitted myeloma or the basic disease recurrence. The average age of presentation described is between 2 and 3 years.
Apart from low backache, this patient did not have other features of multiple myeloma such as lytic bone lesions or fractures. The clinical presentation of myeloma in the study by Sakhuja et al. included backache (28.5%), severe anemia (28.5%), pathological fracture (57.1%), and graft dysfunction due to light chain deposition disease (14.3%).[8]
Because primary EBV infection is a risk factor for PTLD, it is essential to identify patients at risk by performing EBV serology before transplantation. In our center, this is not done as a routine. Patients at the highest risk of PTLD are EBV-seronegative recipients who received EBV-seropositive organs. Although the evidence for prophylaxis against EBV infection in patients with EBV Ig G-negative serology is not very clear, recipients who are EBV IgG-negative may benefit from prophylaxis with ganciclovir or acyclovir. Canadian transplant units recommend ganciclovir prophylaxis for donor positive and recipient negative EBV IgG children as they have not been previously exposed to the primary infection. Although ganciclovir is used for CMV prophylaxis which is used much more extensively and consistently in adults, there may be instances when the drug has to be used for EBV prophylaxis when not indicated for CMV prophylaxis. In a study published by Rocchi et al., a positive correlation between PTLD and number EBV infected cells in peripheral blood was made.[14] Acute EBV infection and infectious mononucleosis usually have 10–2000 copies/ml. In PTLD, the viral load is invariably above 5000 copies/ml.[15] Our patient had 12,567 copies/ml, strongly suggesting a positive correlation between EBV infection and PTLD.
Thus, we recommend routine pretransplant surveillance of all donors and recipients for EBV serology, especially in high risk groups such as second renal transplant who may benefit from prophylaxis to prevent PTLD. Second renal transplantation and heightened immunosuppression for ABO incompatibility could be the reason for the development of EBV-positive multiple myeloma after transplantation. There are no case reports of EBV-positive multiple myeloma after ABO incompatible renal transplant in the literature until date. Viral DNA demonstration in the plasma cells by in situ hybridization for Epstein-Barr coded RNA or immunohistochemistry to detect viral latent membrane protein-1 was not done due to financial constraints.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Dr. Maya Menon – Department of Pathology.
References
- Potent immunosuppression for ABO-incompatible renal transplantation may not be a risk factor for malignancy. Transplant Proc. 2012;44:210-3.
- [Google Scholar]
- Cancer risk after ABO incompatible living-donor kidney transplantation. Transplantation. 2013;96:476–79.
- [Google Scholar]
- The changing pattern of posttransplant malignancies. Transplant Proc. 1991;23(1 Pt 2):1101-3.
- [Google Scholar]
- Cancer incidence in renal transplant patients treated with azathioprine or cyclosporine. Transplant Proc. 1987;19(1 Pt 3):2214-6.
- [Google Scholar]
- Myeloma, Hodgkin disease, and lymphoid leukemia after renal transplantation: characteristics, risk factors and prognosis. Transplantation. 2006;81:888-95.
- [Google Scholar]
- Twenty and 25 years survival after cadaveric renal transplantation. Transplant Proc. 1995;27:2154-5. Mahony JF, Caterson RJ, Coulshed S, Stewart JH, Sheil AG. Twenty and 25 years survival after cadaveric renal transplantation. Transplant Proc 1995;27:2154-5.
- [Google Scholar]
- Spectrum of lymphoproliferative disorders following renal transplantation in North India. Indian J Nephrol. 2013;23:287-91.
- [Google Scholar]
- Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374-85.
- [Google Scholar]
- Long-term T-cell-mediated immunity to Epstein-Barr virus in renal-allograft recipients receiving cyclosporin A. Lancet. 1981;1:10-2.
- [Google Scholar]
- Demonstration of Epstein-Barr virus in a case of multiple myeloma after renal transplantation. Haematologica. 2000;85:773-4.
- [Google Scholar]
- French PTLD Working Group. Post-transplant lymphoproliferative disorders occurring after renal transplantation in adults: report of 230 cases from the French Registry. Am J Transplant. 2006;6:2735-42.
- [Google Scholar]
- Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354-62.
- [Google Scholar]
- Quantitative evaluation of Epstein-Barr-virus-infected mononuclear peripheral blood leukocytes in infectious mononucleosis. N Engl J Med. 1977;296:132-4.
- [Google Scholar]
- Use of quantitative competitive PCR to measure Epstein-Barr virus genome load in the peripheral blood of pediatric transplant patients with lymphoproliferative disorders. J Clin Microbiol. 1997;35:1612-5.
- [Google Scholar]







