Translate this page into:
Rifampicin and anti-hypertensive drugs in chronic kidney disease: Pharmacokinetic interactions and their clinical impact
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Patients on dialysis have an increased incidence of tuberculosis (TB). Rifampicin, a first-line antitubercular therapy (ATT) drug, is a potent inducer of hepatic cytochrome P450 (CYP). There is potential for pharmacokinetic interaction between rifampicin and anti-hypertensives that are CYP substrates: amlodipine and metoprolol. Therefore, hypertensive patients receiving rifampicin-based ATT are at risk for worsening of hypertension. However, this hypothesis has not yet been systematically studied. In this prospective study, hypertensive CKD 5D patients with TB were followed after rifampicin initiation. Blood pressure (BP) was ≤140/90 mmHg with stable anti-HT requirement at inclusion. Serum amlodipine, metoprolol, and prazosin levels were estimated by high-performance liquid chromatography at baseline and 3, 7, 10, and 14 days after rifampicin initiation. BP and anti-HT requirement were monitored for 2 weeks or until stabilization. All 24 patients in the study had worsening of hypertension after rifampicin and 83.3% required increase in drugs to maintain BP <140/90 mmHg. Serial amlodipine levels were estimated in 16 patients; metoprolol and prazosin in four patients each. Drug levels declined by >50% in all patients and became undetectable in 50-75%. Drug requirement increased from 4.5 ± 3.6 to 8.5 ± 6.4 units (P < 0.0001). Mean time to first increase in dose was 6.5 ± 3.6 days. Eleven (46%) patients experienced a hypertensive crisis at 9.1 ± 3.8 days. Three of them had a hypertensive emergency with acute pulmonary edema. In two patients, rifampicin had to be discontinued to achieve BP control. In conclusion, rifampicin caused a significant decrease in blood levels of commonly used anti hypertensives. This decrease in levels correlated well with worsening of hypertension. Thus, we suggest very close BP monitoring in CKD patients after rifampicin initiation.
Keywords
Anti-hypertensive drugs
chronic kidney disease
drug interaction
hypertension
rifampicin
Introduction
Chronic kidney disease (CKD) leads to an immunocompromised state with enhanced susceptibility to infections. The increased incidence of tuberculosis (TB) in patients on maintenance hemodialysis (MHD) was noted as early as the 1970s.[1] It is reported to be 4-15%, which is 6-16 times that of the general population.[234] Further, as majority are hypertensive (HT),[5] it can be inferred that a significant number require simultaneous treatment with antitubercular therapy (ATT) and anti-HT drugs. This clinical scenario is especially relevant in TB endemic countries.
Rifampicin, a first-line antitubercular drug, exhibits pharmacokinetic interactions with numerous drugs. It is a potent inducer of cytochrome P450 (CYP) 3A4 in the liver and small intestine.[6] Rifampicin also induces intestinal and hepatic P-glycoprotein, which function as cellular efflux pumps.[7] Full induction of drug metabolizing enzymes is reached in about 1 week after starting rifampicin treatment and the induction dissipates in approximately 2 weeks after discontinuing rifampicin.[8]
It has been our experience that hypertensive CKD patients with previously controlled blood pressure (BP) develop worsening hypertension after initiation of rifampicin as part of ATT. There is evidence that rifampicin induces anti-HT drug metabolism and decreases their potency. Pharmacokinetic studies in healthy volunteers and case studies of hypertensive patients have described significant interactions of beta-blockers (BB) and calcium-channel blockers (CCB) with rifampicin.[910111213] However, the interaction could be unpredictable in the presence of renal dysfunction due to factors such as decreased renal excretion of anti-HTs, decreased serum rifampicin levels, the tendency for fluid retention, renin-mediated hypertension and clearance of drugs during dialysis.
So far, this issue has not been systematically addressed in the clinical setting. In our study, we aimed to correlate the change in the degree of hypertension, anti-HT drug requirements and serum levels of commonly used anti-HTs in CKD 5D patients initiated on rifampicin-based ATT.
Subjects and Methods
Study design
This was a single-center, prospective observational cohort study conducted at a tertiary care hospital in India between September 2012 and December 2013. Adult (≥18 years) hypertensive CKD 5D patients recently diagnosed with TB and planned for initiation of rifampicin-based daily ATT were screened for eligibility. Patients enrolled had controlled hypertension (BP ≤140/90 mmHg) with stable anti-HT drug requirement for ≥4 weeks before recruitment. All patients were receiving regular thrice-weekly MHD. Patients were excluded if they were receiving concomitant drugs which could influence CYP450 metabolism; had a current history of smoking or alcohol abuse; were inadequately dialyzed; had fluid overload, or were poorly compliant to treatment. The study protocol was approved by the Institutional Ethics Committee and written informed consent was sought from all participants.
Baseline evaluation included a detailed medical and drug history, physical examination, urine analysis, complete hemogram, liver and renal function tests, renal ultrasound, electrocardiography, echocardiography, and assessment of comorbid conditions such as cardiac disease. Serum samples for baseline estimation of anti-HT drug levels were drawn in all patients before start of rifampicin. Baseline levels of particular anti-HT were measured in patients who were receiving the drug for at least 4 weeks prior to recruitment. After the introduction of rifampicin-based ATT, patients were followed regularly for 2 weeks or until stabilization of BP and anti-HT requirement, whichever was longer. Data collected included BP recording, episodes of uncontrolled hypertension (>140/90 mmHg) and change in anti-HT drug prescription with regard to number, dosage, and frequency of medication. Hypertensive crises were defined as per the Joint National Committee 7 guidelines.[14]
Estimation of serum anti-hypertensive drug levels
Serum levels of amlodipine besylate, metoprolol succinate, and prazosin hydrochloride were measured using high-performance liquid chromatography (HPLC) technique. Blood samples for estimation were drawn at baseline (day 0) and days 3, 7, 10, and 14 days after starting rifampicin. Samples were also taken whenever there was uncontrolled hypertension or change in anti-HT drug requirement. Blood was centrifuged and supernatant serum was stored at −80°C until analysis.
The Shimadzu UFLC model HPLC system with fluorescence detector (Shimadzu Corporation, Kyoto, Japan) was used to measure the drug levels. All solvents used were of HPLC-grade while other chemicals were of analytical grade and were obtained for Merck (India). HPLC standards for amlodipine besylate, metoprolol succinate, and prazosin hydrochloride were supplied by Sun Pharma, India. Calibration curves were plotted using five-point calibration standards prepared by plotting peak area against various known concentrations of the drugs. Linear calibration curves were used to estimate drug level in patient samples. The procedure was validated to ensure the suitability and accuracy of the method in terms of linearity of the chromatographic response, accuracy, precision, sensitivity, specificity, and recovery.
Statistical analysis
Outcome parameters assessed were change in BP, anti-HT drug requirement, and serum levels of amlodipine, metoprolol, and prazosin after initiation of rifampicin. Anti-HT drug prescription in terms of number of drugs, dosage, and frequency of administration was computed into unit scores. Minimum dose or increments in dose of individual anti-HTs used was considered as 1 unit equivalent. These were then algebraically added to derive the total anti-HT drug requirement at a given time point. One unit was taken as amlodipine 5 mg, metoprolol succinate or tartarate 25 mg, prazosin XL 2.5 mg, clonidine 0.1 mg, nifedipine SR 20 mg, atenolol 25 mg, ramipril 5 mg, and minoxidil 5 mg.
Categorical variables were summarized by frequency (%) and quantitative variables were expressed as a mean ± standard deviation. Paired t-test was used to compare changes in baseline and post-rifampicin values of quantitative variables. All statistical analyses were performed using SPSS® Statistics version 22.0 (IBM® Corp., Armonk, New York) and P < 0.05 was considered as statistically significant.
Results
Study population
Twenty-four Asian Indian patients on MHD with newly diagnosed TB were recruited for the study. They were all planned for initiation of daily rifampicin-based ATT. Nineteen patients were male (79%) and mean age of the cohort was 41.1 ± 13.1 years (range: 20–74). Mean body mass index was 20.1 ± 3.3 kg/m2(range: 15–27.8) and serum albumin level was 3.9 ± 0.6 g/dl (range: 2.9-5.2). Mean systolic and diastolic BPs at baseline were 132 ± 9 and 82 ± 8 mmHg, respectively with stable anti-HT requirement for at least 4 weeks prior to recruitment. Distribution of various anti-HT drug classes, as well as concomitant medications, are shown in Table 1. Prior to initiation of rifampicin-based ATT, 79% of the study group required ≤2 anti-HT drugs for maintaining BP ≤140/90 mmHg. Most patients (87.5%) required <10 units of anti-HT drugs at baseline and 75% required ≤5 units. Most commonly prescribed drugs were amlodipine, clonidine, metoprolol, and prazosin.
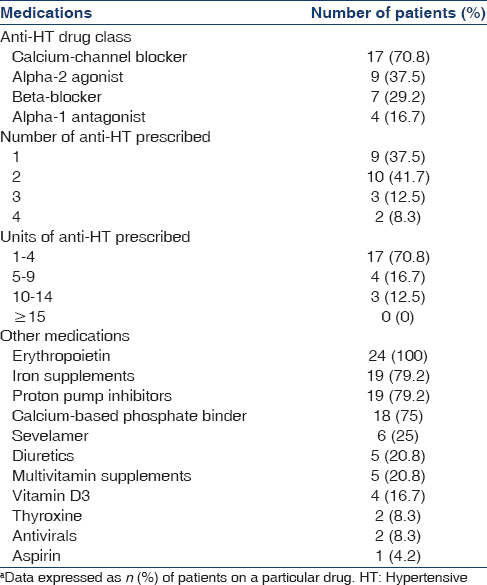
Diagnosis of TB and decision to treat with daily rifampicin-based ATT was per standard clinical practice. All patients were initiated on daily four-drug ATT with isoniazid, rifampicin, pyrazinamide, and ethambutol. The dose of rifampicin used was 450 mg/day for patients weighing ≤60 kg and 600 mg/day for patients >60 kg.
Change in blood pressure and anti-hypertensive drug requirement
After initiation of rifampicin, 20 patients (83.3%) required an increase in anti-HT drugs to maintain BP ≤140/90 mmHg. The overall increase in anti-HT drug requirement from 4.5 ± 3.6 units to 8.5 ± 6.4 units was statistically significant (P < 0.0001) [Figure 1]. The mean time to first increase in anti-HT drugs was 6.5 ± 3.6 (range: 2-14) days. The other four patients also had increase in systolic and diastolic BP by mean of 24 ± 10 mmHg (P = 0.017) and 15 ± 2 mmHg (P = 0.001), respectively, which was both clinically and statistically significant. Eleven of the 24 (46%) patients experienced a hypertensive crisis. Three patients had a hypertensive emergency with the development of acute left ventricular failure and pulmonary edema. The mean time to a crisis was 9.1 + 3.8 days, with a range of 3-14 days.

- Increase in anti-hypertensive requirement (expressed in units) with time after rifampicin initiation
In two patients, BP could not be controlled despite maximal doses of anti-HTs including amlodipine/nifedipine, metoprolol, prazosin, clonidine, minoxidil, and ramipril. They had frequent hypertensive urgencies during hemodialysis, requiring intravenous nitroglycerine infusion, and labetalol. Both these patients had well-controlled BP on three anti-HT drugs prior to initiation of rifampicin. Ultimately, rifampicin was withdrawn and fluoroquinolone was started instead. Three days after withdrawal, the anti-HT requirement began decreasing and by the 10-12th day they were back to requiring only three drugs to maintain BP ≤140/90 mmHg.
Anti-hypertensive drug levels
The serial serum concentrations of amlodipine were available in 16 patients, 5 of whom were on 5 mg/day and the other 11 were on 10 mg/day of amlodipine. With the 5 mg/day dose of amlodipine, baseline levels ranged from 10.8 ng/mL to 392 ng/mL. The median level was 27.6 ng/mL and mean was 109.7 ± 161 ng/mL. In the patients who were on 10 mg/day amlodipine, the baseline serum concentration varied from 7.3 to 332.8 ng/mL with median and mean of 37.7 ng/mL and 95.5 ± 100.8 ng/mL, respectively [Table 2]. There was a decrease in serum amlodipine levels in all 16 patients after initiation of rifampicin [Figure 2]. The mean % decline from the baseline was 81.7 ± 20.6% with a range of 52-100%. In 8 of the 16 patients, the levels became undetectable by HPLC.


- Serial serum amlodipine levels after rifampicin initiation
Of four patients on metoprolol succinate, 2 were receiving 50 mg/day and one each was receiving 25 mg/day and 100 mg/day. The baseline serum concentrations of metoprolol were 27.2 ng/mL (25 mg/day), 45.9 ng/mL and 84 ng/mL (50 mg/day) and 49.5 ng/mL (100 mg/day) [Table 2]. Unlike amlodipine, the decrease in serum metoprolol level was not a steady decline. All four patients however, had a decrease in drug concentration from the baseline [Figure 3]. The mean % decline was 91.9 ± 16.2% (range: 67.6-100%) and three patients (75%) had decline to undetectable levels.

- Serial serum metoprolol levels after rifampicin initiation
Prazosin was prescribed to five patients prior to initiation of rifampicin and serum levels estimated in 4 of them. Two patients were on 5 mg/day and one each was receiving 10 mg/day and 15 mg/day. The baseline serum concentrations of prazosin varied markedly [Table 2]. They were 7.9 ng/mL and 4208.3 ng/mL (5 mg/day), 4398.6 ng/mL (10 mg/day), and 923.2 ng/mL (15 mg/day). Although prazosin and rifampicin are not described to interact pharmacokinetically, we observed a decrease in drug concentration from baseline in all four patients [Figure 4]. Levels declined by 98.1 ± 2.7% (range: 94.3–100%) and became undetectable in two patients (50%).

- Serial serum prazosin levels after rifampicin initiation
Discussion
Ours is a systematic study to address the important issue of interaction of rifampicin with antihypertensive medications in CKD patients. All 24 CKD-5D patients experienced worsening hypertension after initiation of rifampicin-based ATT and twenty patients had a significant increase in their anti-HT drug requirement. It is also noteworthy that eleven patients suffered a hypertensive crisis and in two, rifampicin ultimately had to be stopped to achieve BP control.
In the only other study on this issue, Sharma et al. analysed the effect of antitubercular medications on BP control in a predialysis CKD cohort.[15] They compared 62 CKD patients with TB with 73 CKD controls. They also observed an increase in anti-HT medications in 60% of patients in the TB group and a two-fold increase in drug requirement (P < 0.0001). They inferred that the temporal relationship between starting antitubercular medications and increase in anti-HT medications suggested a cause and effect relationship. However, they did not perform pharmacokinetic profiling as in our study.
Serum levels of anti-HTs were serially estimated in our study and the decline of drug level correlated clinically with increased BP. The variations in the baseline concentrations of the drugs may be attributed to difference in time of sampling with respect to drug ingestion, meals or hemodialysis and individual patient characteristics. However, each patient in our study served as his own control and we attempted to circumvent this problem by drawing the sample at the same time each day prior to the hemodialysis session.
All patients showed a decline in serum amlodipine and metoprolol concentrations. Studies in healthy volunteers have shown that rifampicin decreases the bioavailability, plasma levels, and pharmacological effects of beta-blockers and calcium channel blockers by induction of CYP2D6 and CYP3A4, respectively. Bennet PN et al. reported that rifampin reduced the plasma concentrations of metoprolol causing a 33% mean decrease in the area under the curve.[9] Within 15 days after discontinuation, these changes had reverted to the initial values. Similarly, Holtbecker et al. demonstrated decreased bioavailability of oral nifedipine from 41.2% to 5.3% after 7 days of rifampin.[12] Exacerbation of angina and development of uncontrolled hypertension after rifampicin in patients previously well-controlled on nifedipine have also been reported by Tsuchihashi et al.[11] and Tada et al.,[13] respectively. As far as prazosin is concerned, there is no known interaction described with rifampicin in literature. However, we observed a significant decrease in serum prazosin levels with rifampicin. Thus, this interaction warrants further investigation.
Two of our patients actually required withdrawal of rifampicin due to uncontrolled hypertension despite maximal anti-HT drug prescription. After substitution of rifampicin with a fluoroquinolone, their anti-HT requirement decreased back to baseline in 10-12 days. Singh described a similar occurrence in two dialysis-dependent patients 14-15 days after initiation of rifampicin.[16]
Though small in terms of number of cases, the strength of our study is that it is well-designed, prospective, and the first study to demonstrate the pharmacokinetic effects and clinical significance of the interaction of rifampicin with anti-HTs in patients with renal dysfunction. It provides a proof of concept that rifampicin induces the metabolism of anti-HT drugs, resulting in clinically significant increases in BP. This interaction may be of increased significance in the setting of CKD, and leads to a high incidence of hypertensive crises. In extreme situations, when BP remains uncontrolled despite maximal anti-HT drugs, discontinuation of rifampicin may be the only feasible option. Whether administration of supramaximal doses of anti-HT drugs with rifampicin would counteract the pharmacokinetic interaction and help in better control of hypertension needs further study.
There are three clear messages from our study for handling such clinical situations. First, avoid rifampicin in patients of CKD with hypertension as far as possible. Caution needs to be exercised in patients with poorly controlled BP and fluoroquinolones may be used instead of rifampicin. Second, if rifampicin is being used, it is essential to ensure strict BP monitoring. This is especially so in first 2 weeks, when there is maximum induction of liver enzymes. Third, patients as well as the primary care physicians must be made aware of the potential risk of uncontrolled hypertension or a hypertensive crisis and should be more watchful in patients at risk for these emergencies.
The limitations of our study are small sample size and single centre study in a specific subgroup of CKD 5D patients. However, we have no reason to believe that similar findings will not be there in milder degree of CKD. Further, there is a caveat to conversion of anti-HT drugs to unit scores. For example, 1 unit of amlodipine (5 mg) might not be equivalent to 1 unit of clonidine (0.1 mg). Finally, we did our best to enforce compliance with frequent counseling; however, variation of drug and dietary compliance in the same patient with time may also contribute to change in BP.
Conclusion
Rifampicin is a potent inducer of cytochrome p450 enzymes and decreases serum levels of commonly used antihypertensive drugs – amlodipine, metoprolol, and prazosin. This interaction is of significant clinical importance in hypertensive CKD 5D patients initiated on rifampicin-based antitubercular treatment. They experience significant lowering of anti-HT drug levels and corresponding worsening of hypertension. Given the clinical impact of our findings and ease of applicability, we feel it would be prudent to monitor patients closely for worsening of hypertension after initiation of rifampicin or use an alternative antitubercular drug in place of rifampicin.
Financial support and sponsorship
Intramural Research Grant from All India Institute of Medical Sciences, New Delhi.
Conflicts of interest
There are no conflicts of interest.
References
- Tuberculosis in patients undergoing maintenance hemodialysis. Am J Med. 1979;67:597-602.
- [Google Scholar]
- Tuberculosis in maintenance haemodialysis patients. Study from an endemic area. Postgrad Med J. 1981;57:492-8.
- [Google Scholar]
- Hypertension and CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), 1999-2004. Am J Kidney Dis. 2008;51(4 Suppl 2):S30-7.
- [Google Scholar]
- Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: Studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299:849-57.
- [Google Scholar]
- Pharmacogenetics of the human drug-transporter gene MDR1: Impact of polymorphisms on pharmacotherapy. Drug Discov Today. 2001;6:835-9.
- [Google Scholar]
- Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin Pharmacokinet. 2003;42:819-50.
- [Google Scholar]
- Effect of rifampicin on metoprolol and antipyrine kinetics. Br J Clin Pharmacol. 1982;13:387-91.
- [Google Scholar]
- Induction of propranolol metabolism by rifampicin. Br J Clin Pharmacol. 1983;16:565-9.
- [Google Scholar]
- A case of variant angina exacerbated by administration of rifampicin. Heart Vessels. 1987;3:214-7.
- [Google Scholar]
- The nifedipine-rifampin interaction. Evidence for induction of gut wall metabolism. Drug Metab Dispos. 1996;24:1121-3.
- [Google Scholar]
- Case report: Nifedipine-rifampicin interaction attenuates the effect on blood pressure in a patient with essential hypertension. Am J Med Sci. 1992;303:25-7.
- [Google Scholar]
- The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560-72.
- [Google Scholar]
- Effect of antitubercular medications on blood pressure control in chronic kidney disease patients with tuberculosis: A prospective cohort study. J Nephrol. 2006;19:771-7.
- [Google Scholar]
- Uncontrolled blood pressure in tubercular patients on hemodialysis: Think rifampicin. Hemodial Int. 2012;16:324-5.
- [Google Scholar]







