Translate this page into:
Septicemic melioidosis in a transplant recipient causing graft dysfunction
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Melioidosis, an infectious disease caused by Burkholderia pseudomallei, is endemic in the Northern Australia and South-East Asia. It is an emerging disease in the Indian subcontinent. Melioidosis tends to run a potentially lethal course in immunocompromised individuals and data in renal transplantation are scarce. The clinical presentation of melioidosis is diverse, mimicking several other infectious diseases. Diagnosis could be delayed in transplant recipients. Choice and duration of antimicrobial therapy, management of immunosuppression, and patient and graft outcomes are other issues to be addressed. We report septicemic melioidosis with pulmonary involvement in a 32-year old renal transplant recipient that caused acute allograft dysfunction.
Keywords
Allograft dysfunction
melioidosis
renal transplantation
septicemia
Introduction
Melioidosis, an infectious disease caused by Burkholderia pseudomallei, has been reported sporadically from various parts of India.[123] Data in renal transplant recipients are scarce.[456] We report a case of septicemic melioidosis in a renal transplant recipient presenting with pneumonia, mediastinal lymphadenopathy, and graft dysfunction.
Case Report
A 32-year-old man underwent preemptive kidney transplantation for diabetic nephropathy. He received a kidney from his mother. He had 4/6 human leukocyte antigen match, negative cross match and received no induction. He received triple-drug immunosuppression with tacrolimus (trough level 9.1 ng/ml at the fifth posttransplant month), azathioprine 2 mg/kg/day, and prednisolone 10 mg/day. He manifested gastrointestinal intolerance to both mycophenolate and enteric coated mycophenolic acid, and hence azathioprine was chosen. He had completed vaccination against hepatitis B, pneumococcus, and varicella-zoster prior to transplant. Cotrimoxazole prophylaxis was excluded due to the previous hypersensitivity. His allograft functioned normally (serum creatinine 1.2 mg/dl) until the fifth posttransplant month when he presented with continuous high-grade fever with chills and rigor and dry cough of 6 days duration. His vitals initially remained stable. Apart from pyrexia, clinical examination was unremarkable.
Investigations were notable for neutrophilic leukocytosis with leftward shift and allograft dysfunction. Serum creatinine was 1.5 mg/dl at the presentation. Blood and urine culture grew no organisms. Blood film was negative for malarial parasite. Ultrasonogram and Doppler study of allograft were unremarkable. Chest radiograph [Figure 1] revealed superior mediastinal widening. Computerized tomography of chest [Figures 2 and 3] revealed subpleural nodules in the right upper lobe, some of them showing cavitation and extensive mediastinal lymphadenopathy involving para- and pre-tracheal, carinal, and bilateral hilar nodes [Figure 4]. Endobronchial ultrasound guided needle aspiration of mediastinal nodes yielded purulent material rich in neutrophils. The lymph node aspirate and bronchoalveolar lavage sample did not yield any specific organism upon staining and culture.
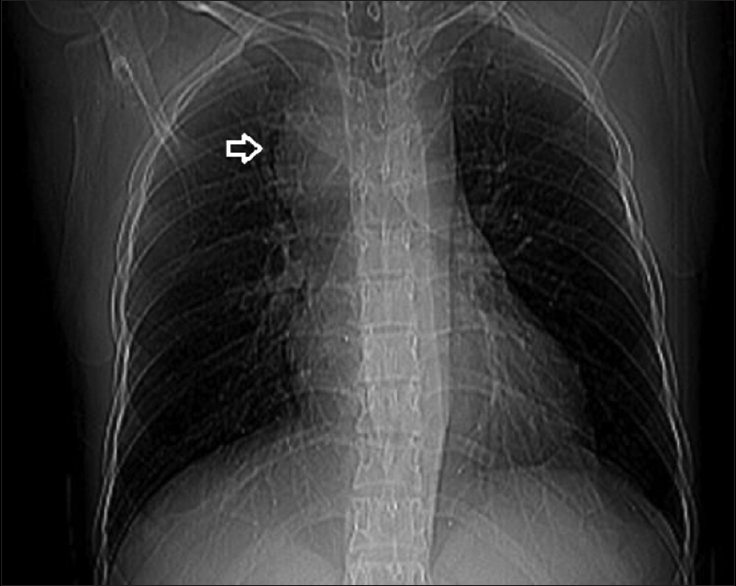
- Chest radiograph revealing superior mediastinal widening

- Computed tomography thorax axial section – arrows point to subpleural nodules
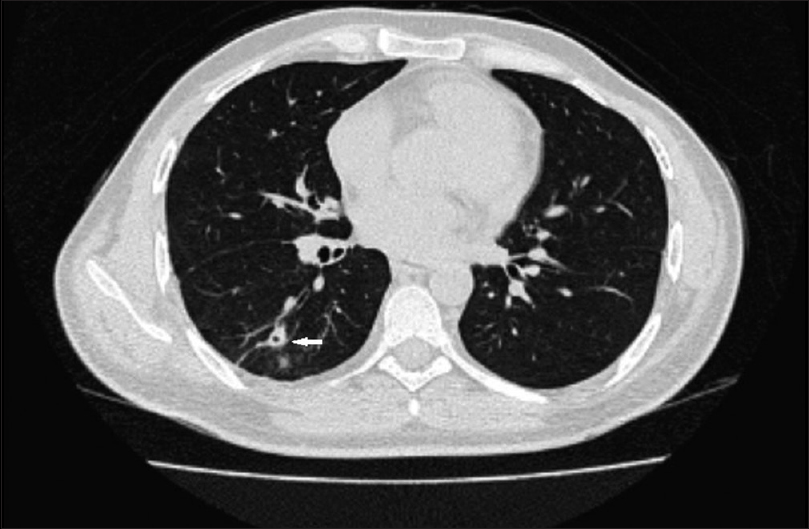
- Computed tomography thorax axial section – arrow points to a cavitating nodule

- Computed tomography thorax revealing extensive mediastinal lymphadenopathy
He was given empiric antimicrobial coverage for community-acquired pneumonia with intravenous ceftriaxone and oral levofloxacin. He continued to have fever and was prescribed new regimens which included piperacillin tazobactam, meropenem, and vancomycin. He continued to have fever and progressed to sepsis with hypotension. Serum creatinine increased to 1.9 mg/dl in the 4th week of illness. Immunosuppression was reduced – azathioprine was stopped, and tacrolimus dose reduced to maintain a trough level of 7.2 ng/ml. He was resuscitated, and allograft biopsy was performed which revealed acute tubular injury. By the 4th week of illness, he began to expectorate sputum the culture of which grew B. pseudomallei. Melioidosis was diagnosed and intravenous ceftazidime was initiated. Fever remitted on the 5th day after starting treatment. Since he was allergic to cotrimoxazole, he was treated with oral doxycycline for eradication therapy. He remained afebrile at the end of three months of eradication therapy with good allograft function (serum creatinine 1.3 mg/dl).
Discussion
Fever and allograft dysfunction have a wide range of differentials in transplant recipients. This patient presented with high-grade continuous fever with chills and rigor, pneumonia with cavitating nodules, and extensive mediastinal lymphadenopathy. He failed to respond to empiric antibiotic therapy for community-acquired pneumonia. He progressed to sepsis syndrome with hypotension and graft dysfunction. Initial considerations included reactivation tuberculosis (in lieu of past tuberculous pleural effusion) with dissemination, fungal infections such as aspergillosis, cryptococcosis, and bacterial infections such as staphylococcal pneumonia which could cavitate. Although posttransplant lymphoproliferative disorder (PTLD) was a diagnostic possibility based on the findings of imaging studies,[7] acute presentation with high grade continuous fever and progression to sepsis made PTLD unlikely. Staphylococcal pneumonia presents with thin-walled cavities distended with air (pneumatoceles)[8] while the patient had thick walled cavities. Blood culture grew none and sputum culture done in the 4th week of illness isolated B. pseudomallei.
Melioidosis is endemic in the Northern Australia and South-East Asia.[9] It is being increasingly recognized in India.[123] There is a paucity of information regarding the magnitude of this infection in the different regions of India. The causative agent B. pseudomallei, a Gram-negative aerobic bacillus, is a saprophyte found in the soil and surface water. It is a facultative intracellular organism transmitted by inhalation, ingestion, and cutaneous inoculation.[10] The infection can result in the clinical disease or a latent infection.[10] Melioidosis in transplant recipients could be due to primary infection or reactivation of latent infection. The clinical presentation could be acute, subacute, or chronic.
Pneumonia is the most common clinical presentation of patients with melioidosis.[10] Acute melioidosis pneumonia has a spectrum ranging from fulminant septic shock with high mortality to mild pneumonia with little mortality. On chest radiographs, diffuse nodular infiltrates often develop throughout both lungs with predilection for upper lobes. The nodules may coalesce and cavitate due to caseous necrosis.[10] Mediastinal adenopathy could occur.[11]
Diabetes mellitus, alcoholism, renal failure, and steroid therapy predispose to severe forms of melioidosis such as septicemic and pneumonic forms.[10] Predisposition in these situations reflects impairment of neutrophil and other phagocytic cell functions.[12]
Diagnosis is based on isolation in culture[13] or by serological investigations[141516] such as indirect hemagglutination, or enzyme-linked immunosorbent assay. B. pseudomallei readily grows in commercial culture media but could be mistaken for Pseudomonas species.[13] Polymerase Chain Reaction assay of buffy coat could be used to detect this organism in febrile patients.[17] Treatment recommendation[10] for adults consists of initial intensive therapy for at least 14 days with any one of intravenous ceftazidime, meropenem, or imipenem followed by eradication therapy for minimum of three months with cotrimoxazole or doxycycline. It may take a prolonged period of fever defervescence (mean 12 days in a South Indian study),[18] and hence antibiotics need not be transitioned prematurely.
The patient in this report had documented hypersensitivity to cotrimoxazole which precluded routine prophylaxis with the drug. Whether cotrimoxazole prophylaxis could prevent melioidosis in transplant recipients needs to be answered. Even though empiric regimen included Meropenem, to which he might have responded, he was prematurely transitioned to another antibiotic. After a diagnosis of melioidosis, he was treated with intravenous ceftazidime for 15 days. Diabetes mellitus and immunosuppressive therapy predisposed him to life threatening melioidosis.
Conclusion
Transplant recipients are prone to develop life-threatening forms of melioidosis such as pneumonic and septicemic forms. Allograft dysfunction could result from sepsis and hypotension. Prompt consideration of this infection in transplant recipients in endemic regions in the setting of fever, pneumonia, lymphadenopathy, visceral abscess, and sepsis could facilitate early diagnosis, institution of specific antimicrobial therapy, and fasten recovery. Since it may take a long time for defervescence of fever even with appropriate antimicrobial therapy, establishing a diagnosis of melioidosis will help to avoid premature withdrawal of effective antimicrobial drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Melioidosis: An emerging infection in India. J Assoc Physicians India. 2013;61:612-4.
- [Google Scholar]
- Melioidosis in southern India: Epidemiological and clinical profile. Southeast Asian J Trop Med Public Health. 2010;41:401-9.
- [Google Scholar]
- Expect the unexpected: Pleuro-pulmonary melioidosis in a renal transplant recipient. Transpl Infect Dis. 2013;15:E40-3.
- [Google Scholar]
- Transplantation and tropical infectious diseases. Int J Infect Dis. 2010;14:e189-96.
- [Google Scholar]
- CT findings in posttransplantation lymphoproliferative disorder of renal transplants. AJR Am J Roentgenol. 2000;175:183-8.
- [Google Scholar]
- Radiographic features of staphylococcal pneumonia in adults and children. Thorax. 1996;51:539-40.
- [Google Scholar]
- Melioidosis: Epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383-416.
- [Google Scholar]
- Burkholderia pseudomallei and Burkholderia mallei: Melioidosis and Glanders. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (7th ed). Philadelphia: Churchil Livingstone Elsevier; 2010. p. :2869-80.
- [Google Scholar]
- Pulmonary melioidosis: Clinical-radiologic correlation in 183 cases in northeastern Thailand. Radiology. 1988;166:711-5.
- [Google Scholar]
- A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J Infect Dis. 2007;195:99-107.
- [Google Scholar]
- Identification of Pseudomonas pseudomallei in clinical practice: Use of simple screening tests and API 20NE. J Clin Pathol. 1989;42:645-8.
- [Google Scholar]
- Recent developments in laboratory diagnosis of melioidosis. Acta Trop. 2000;74:235-45.
- [Google Scholar]
- Indirect hemagglutination assay in patients with melioidosis in northern Australia. Am J Trop Med Hyg. 2006;74:330-4.
- [Google Scholar]
- Accuracy of enzyme-linked immunosorbent assay using crude and purified antigens for serodiagnosis of melioidosis. Clin Vaccine Immunol. 2007;14:110-3.
- [Google Scholar]
- A multiplex nested PCR for the simultaneous detection of Salmonella typhi, Mycobacterium tuberculosis, and Burkholderia pseudomallei in Patients with Pyrexia of Unknown Origin (PUO) in Vellore, South India. Mol Diagn Ther. 2014;18:315-21.
- [Google Scholar]
- Septicaemic melioidosis in a tertiary care hospital in south India. Indian J Med Res. 2003;117:119-21.
- [Google Scholar]







