Translate this page into:
Successful Treatment of Multiple Angiomyolipomas with Sirolimus in a Child
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tuberous sclerosis complex frequently results in the formation of renal angiomyolipomas (AMLs). Sirolimus (SIR) or everolimus can be used to treat large AMLs, although this treatment has rarely been described in children, particularly for multiple renal AMLs. A 15-year-old girl presented with bilateral severe chronic flank pain coinciding with increased renal size and hundreds of renal AMLs. We opted to treat her with SIR over the course of 3.5 years. Following her treatment, her renal size had substantially decreased and the AMLs had shrunk. The patient's pain subsided as well. Our case, which has never been described in published literature, demonstrates that a child with multiple renal AMLs can be treated with SIR, and suggests that this treatment may be a viable option for preventing the development of secondary morbidities such as chronic pain.
Keywords
Chronic pain
everolimus
pediatrics
renal angiomyolipomas
sirolimus
tuberous sclerosis complex
Introduction
Children with tuberous sclerosis complex (TSC) frequently develop angiomyolipomas (AMLs). Most of the children only require observation; however, if AMLs are large or symptomatic, they may be embolized or resected. There was no systemic treatment available until 2008, at which point mTOR inhibition was introduced as a viable treatment option.[12] Although the treatment has rarely been used in children,[3] either sirolimus (SIR) or everolimus (EVR) is recommended if the AMLs are larger than 3.0 cm.[24] There have been no documented cases detailing the use of SIR or EVR to treat numerous AMLs causing substantial renomegaly and chronic pain. Here, we describe a case of a patient with renal AMLs whose kidneys returned to a normal size following treatment with SIR for 3.5 years.
Case Report
An 8-year-old girl antenatally diagnosed with TSC presented with numerous bilateral renal AMLs. The length of her left kidney was 1.6 standard deviations above the norm (z-score = +1.6 [Figure 1]). She was asymptomatic until she developed bilateral severe chronic flank pain at the age of 15. The pain coincided with a renal length of over 13 cm; the z-score for the length of her left kidney was +5.04 [Figure 1]. Her pain was considered secondary to the capsular pain emanating from the significant growth of hundreds of renal AMLs, the largest measuring 3.5 cm in diameter. Her baseline pain was 6/10 and 10/10 at its worst, causing frequent emesis and the inability to sleep. The pain neither subsided nor lessened with the use of strong analgesics such as opioids. An extensive workup did not reveal any other possible etiologies for the chronic pain.
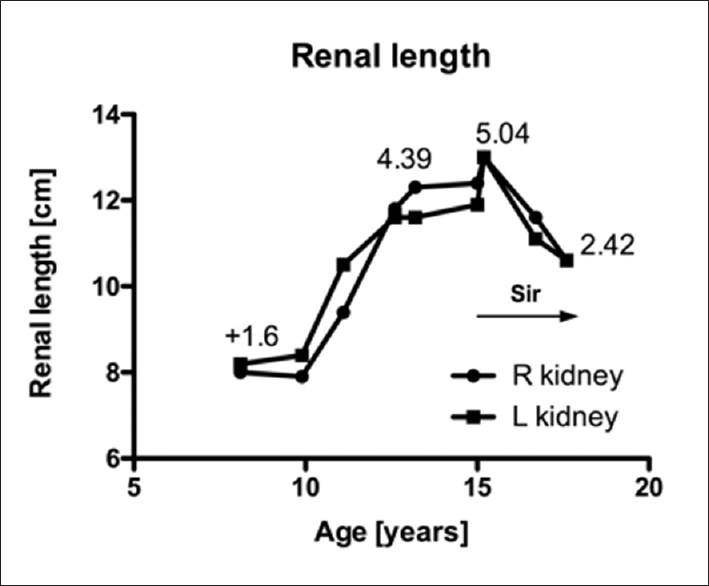
- The evolution of the patient's renal length for both kidneys. The figure includes the z-scores of the left kidney. Sir = Sirolimus
She was conscious and oriented on examination and did not exhibit any acute distress. Her affect was depressed secondary to the chronic pain. Her blood pressure was 103/56 mmHg (50th percentile for her height 112/64 mmHg) and her heart rate was 54/min. She had some tubers around her nose. An abdominal examination revealed bilateral flank tenderness on palpation. An abdominal ultrasound showed numerous AMLs and enlarged kidneys (right kidney: 13.4 cm and left kidney: 12.4 cm [Figure 1]). Her kidney function was stable with a serum creatinine of 72 μmol/L and a cystatin C estimated glomerular filtration rate of 119 mL/min/1.73 m2.[5] Testing did not reveal any hematuria or proteinuria.
SIR was started at a dose of 0.03 mg/kg (2 mg taken once daily) with a target SIR level of 6 – 9 ng/mL (via immunoassay) or 5 – 8 ng/mL (via mass spectrometry), conforming with renal transplant guidelines.[6] Unfortunately, another physician prescribed a macrolide and the drug interaction caused severe stomatitis and a maculopapular rash on her legs, which are adverse effects associated with SIR. She also developed significant emesis and fatigue. The dose of SIR was decreased to 1 mg once daily to minimize these effects.
The dose was continually adjusted over the course of 3 years to minimize ongoing adverse effects, primarily stomatitis. The highest dose she received was 4 mg once daily (0.05 mg/kg). Although her SIR levels were consistently maintained between 2 and 5 ng/mL, the highest measured level was 7.8 ng/mL. Her renal length decreased bilaterally to 10.6 cm, and the z-score for the length of her left kidney decreased to +2.42 [Figure 1]. At her last follow-up appointment, the largest lesion in her right kidney measured 2.8 cm × 3.1 cm × 2.4 cm and the largest lesion in her left lower pole measured 2.2 cm × 1.7 cm × 2.2 cm. Importantly, the patient experienced clinical improvement in her chronic pain. Still, despite successful medical treatment, her pain-associated depression did not subside, and consultations at a pain clinic and with a psychiatrist were necessary to emphasize the importance of employing a multidisciplinary approach to treat her depression.
Treatment with EVR was considered because of its potential to maintain a higher therapeutic exposure while reducing adverse effects; at 10 times the price of SIR, the medication's cost prevented the switch. Of note, neither SIR nor EVR have any published recommended target levels to treat renal AMLs, and previous studies examining treatment with EVR or SIR for tumors have titrated the dosing based on their effect or tolerability.[17]
Discussion
This case demonstrates that SIR is a viable treatment option for numerous renal AMLs causing renal enlargement and capsular pain, which would otherwise not be amenable to any therapy. Overall, further research is necessary to develop precise dosing guidelines since the therapeutic goal published in transplant literature may be too high. This case also raises the question of whether treatment with SIR should be considered early in the course of the disease before the patient develops any secondary morbidity such as chronic pain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140-51.
- [Google Scholar]
- Review of the tuberous sclerosis renal guidelines from the 2012 consensus conference: Current data and future study. Nephron. 2016;134:51-58. Epub ahead of print
- [Google Scholar]
- Sirolimus and tuberous sclerosis-associated renal angiomyolipomas. Arch Dis Child. 2010;95:391-2.
- [Google Scholar]
- International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: Recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:255-65.
- [Google Scholar]
- Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981-5.
- [Google Scholar]
- Pharmacokinetics of mycophenolate mofetil and sirolimus in children. Ther Drug Monit. 2008;30:138-42.
- [Google Scholar]
- The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: Subgroup results from the randomized, placebo-controlled, phase 3 trial EXIST-1. Nephrol Dial Transplant. 2014;29:1203-10.
- [Google Scholar]







