Translate this page into:
Collapsing Glomerulopathy- A Troublemaker for the Renal Allograft: Lessons Learnt
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Collapsing glomerulopathy (CG) is a well-recognized distinct morphological pattern of proliferative parenchymal injury leading to rapid graft failure. We conducted a single-center retrospective study to evaluate the prevalence, clinicopathological features, and prognosis of CG in renal transplant recepient. We analyzed 2518 renal allograft biopsies performed from 2007 to 2015 and correlated their clinicopathological features. The prevalence of CG was 0.83% (21 out of 2518) of allograft biopsies with a higher prevalence of 1.4% during the period from 2012 to 2015. Out of 21 patients, 18 (85.71%) patients had undergone live donor and 3 (14.28%) patients had undergone deceased donor renal transplant. Hypertension was observed in 3 (14.28%) patients. The mean duration of diagnosis for CG was 1.85 ± 1.91 years. Urinalysis revealed microhematuria in 5 (23.8%) patients. The mean 24 h urinary protein excretion was 4.77 ± 5.3 g and serum creatinine was 2.12 ± 1.5 mg/dl. The predominant native kidney diseases in recipients were chronic glomerulonephritis of unknown etiology in 12 (57.14%) patients and hypertensive nephropathy in 3 (14.28%) patients. CG was associated with rejection in 9 (42.85%), calcineurin-inhibitor toxicity in 2 (9.5%), and BK virus nephropathy in 1 patient. All patients received standard triple immunosuppression. Eleven (52.38%) patients developed graft failure over a mean period of 2.2 ± 1.7 years and 6 (28.57%) patients recovered with stable graft function. CG can coexist with viral infection, drug toxicity, rejection, microvascular injury, etc. CG usually presents with moderate to severe proteinuria and may lead to rapid graft dysfunction and subsequent graft failure in most of the patients.
Keywords
Collapsing glomerulopathy
end-stage renal disease
hypertension
podocyte
proteinuria
Introduction
Collapsing glomerulopathy (CG) was first described by Weiss et al. as a distinct variant of focal segmental glomerulosclerosis (FSGS) with progressive renal failure and pathological changes characterized by segmental or global capillary collapse, visceral epithelial cell hypertrophy and hyperplasia with hyaline droplets, and extensive tubulointerstitial inflammation.[1] Clinically, CG is characterized by nephrotic syndrome (NS) with poor response to therapy, rapidly progressing to end-stage renal disease.[23] CG was known to be associated with human immunodeficiency virus (HIV) infection. However, Detwiler et al. reported CG for the first time in 1994 in patients who were HIV negative.[4] CG in a renal allograft can present as recurrent or as a de novo disease. We conducted this single-center retrospective study to evaluate the prevalence, clinicopathological features, and prognosis of CG in renal transplant.
Materials and Methods
We analyzed renal allograft biopsies performed in our center from 2007 to 2015. All biopsies were performed by nephrologists under ultrasound guidance using 16-gauge renal biopsy needle and subjected to light microscopy. All the slides were examined by three pathologists and were reported according to Banff classification. Immunofluorescence (IF) studies were undertaken in a subset of patients where de novo or recurrent disease was suspected clinically. Electron microscopy was not performed due to its nonavailability. For light microscopy, 3 μm thick paraffin sections were stained for hematoxylin and eosin, periodic acid–Schiff, Jone's methenamine silver, and Gomori's trichrome stains. Cryostat frozen sections were subjected to IF studies using antihuman IgG, IgA, IgM, C3, C1q, and fibrinogen antisera (MP Biomedical, France). C4d antibodies were tested by immunohistochemistry using polyclonal antihuman C4d antisera (BioGenex, CA, USA). Tests for antinuclear antibody, anti-double-stranded deoxyribonucleic acid, anti-neutrophil cytoplasmic antibodies (by enzyme-linked immunosorbent assays [ELISA]), and complement components (C3 and C4) were recorded in pertinent cases. ELISA for HIV and hepatitis B and C virus was also carried out.
Demographics included evaluation for donor and recipient age, gender, human leukocyte antigen match, original disease causing renal failure, disease duration, hypertension, serum creatinine (SCr) (mg/dl), 24 h urinary protein loss (g/24 h), and urinalysis. Hypertension was defined as blood pressure >140/90 mmHg and/or ongoing antihypertensive medication. NS was defined as edema, nephrotic-range proteinuria (>40 mg/m²/h on timed sample, spot albumin to creatinine ratio >2 mg/mg), and hypoalbuminemia (<2.5 g/dl). Adequacy of graft biopsies was defined according to Banff criteria. A biopsy with ten viable glomeruli and two arteries in a sample was considered to be adequate.[5] CG was defined morphologically if at least one glomerulus revealed segmental or global collapse of the glomerular capillary tuft with hyperplasia and hypertrophy of visceral epithelial cells. A total number of glomeruli with percentage of globally/segmentally collapsed capillary tufts were reported. Associated involvement of tubulointerstitial compartment in the form of active interstitial inflammation/fibrosis, tubular atrophy, and microcystic dilatation was reported as percentage of cortical area involved. Tubular atrophy was graded as ct1, ct2, and ct3 if ≤25%, 26%–50%, and >50% of the cortex revealed atrophy, respectively. Similarly, interstitial fibrosis was graded as ci1, ci2, and ci3 if ≤25%, 26%–50%, and >50% of the cortex showed fibrosis, respectively.
All the patients received the standard triple drug immunosuppression according to KDIGO clinical practice guidelines.[6]
Statistical analysis was performed and continuous data were expressed as mean ± standard deviation, non-continuous data were expressed in percentage and numerical values.
Results
Twenty-one (0.83%) biopsies showed features of CG out of 2518 renal allograft biopsies performed from 2007 to 2015. However, a higher prevalence of CG, 1.4% was observed during the period from 2012 to 2015. Out of these 21 patients, 18 (85.71%) patients had undergone live donor and 3 (14.28%) patients had undergone deceased donor renal transplant. Males were predominantly affected (male:female 20:1). The mean age of patients was 35 ± 3.65 years. CG was recorded at a mean interval of 1.85 ± 1.91 years posttransplant. Hypertension was noted in 3 (14.28%) patients. Urinalysis revealed microscopic hematuria in 5 (23.8%) patients. The mean 24 h urinary protein excretion was 4.77 ± 5.3 g, and mean SCr was 2.12 ± 1.5 mg/dl [Table 1]. All the patients were serologically nonreactive for HIV, hepatitis B surface antigen, and hepatitis C virus. The predominant native kidney diseases leading to chronic renal failure were chronic kidney disease (CKD) of unknown origin in 12 (57.14%), hypertensive nephropathy in 3 (14.28%) followed by 1 patient each of diabetic nephropathy, sarcoidosis, pauci-immune crescentic glomerulonephritis (GN), C3 glomerulopathy, solitary kidney with CGN, and IgA nephropathy [Table 2]. All the renal biopsies were adequate for interpretation with mean number of glomeruli being 12.43 ± 5.39. The mean number of glomeruli that revealed global/segmental capillary collapse was 3.81 ± 4.29 [Table 3]. Associated focal global sclerosis was observed in 3.25 ± 1.04 glomeruli. Tubular microcystic dilatation was noted in 6 (28.57%) biopsies. Tubular atrophy was graded as ct1 in 15 (71.43%), ct2 in 3 (14.28%), and ct3 in 3 (14.28%) biopsies and interstitial fibrosis as ci1 in 14 (66.67%) biopsies, ci2 in 6 (28.57%), and ci3 in 1 (4.76%) [Table 4]. Vascular changes were noted in 9 (42.8%) patients. Out of nine, five had mild arteriosclerosis, two had nodular subintimal hyalinosis, and two had mucoid intimal proliferation with fibrinoid necrosis of vessel wall suggestive of thrombotic microangiopathy (TMA).




CG alone was reported in 7 (33.3%) patients [Figure 1a], and CG associated with rejection was noted in 9 (42.85%) patients [Figure 1b]. Out of these 9 patients, acute T-cell rejection (TCR) + antibody-mediated rejection (AMR) was observed in 4 (19%), AMR in 4 (19%), and acute TCR in 1 (4.76%) case. AMR was noted in the form of glomerulitis, peritubular capillaritis along with linear C4d deposits across the peritubular capillary membranes, whereas TCR was reported according to tubulitis, intimal arteritis, and interstitial infiltration. CG with acute TMA was reported in 2 (9.5%) patients. Two (9.5%) patients with CG associated with calcineurin inhibitor (CNI) toxicity revealed nodular subintimal hyalinosis with stripped pattern of interstitial fibrosis, and tubular atrophy. One patient also had associated BK virus (BKV) nephropathy.
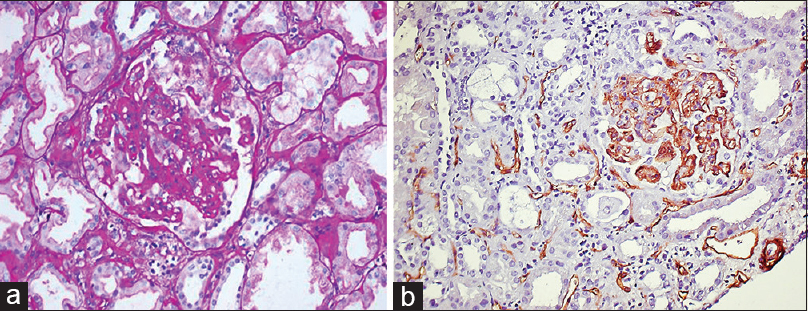
- (a) Glomerular collapse with hyperplasia/hypertrophy of podocytes (periodic acid–Schiff, ×400). (b) Collapsing glomerulopathy and acute antibody-mediated rejection (antihuman C4d antisera, ×200)
IF study was performed in five (23.8%) cases, of which one case showed coarse granular deposits of complement C3 of +3 intensity, along the capillary wall consistent with diagnosis of recurrent C3 glomerulopathy. The second case revealed granular deposits of IgG, IgM and C3 of +2 intensity across the capillary walls and segmental mesangial region, consistent with de novo immune complex glomerulopathy (primary disease was pauci-immune GN). The third case showed granular capillary wall staining of +2 intensity with antihuman IgG antiserum only, and the remaining two cases showed the absence of any immune deposits (native renal primary disease was CKD of unknown origin in these three cases).
All the patients continued to follow the standard triple drug immunosuppression i.e., prednisolone 20 mg/day, Tacrolimus 3.5 mg/day and Mycophenolate sodium 360 mg four times a day. Even in cases with CNI toxicity the patients were kept on triple immunosuppression with standard dose. About sixteen patients underwent plasmapheresis. Plasmapheresis was performed on Cobe Spectra version 7 (Gambro China), on alternate days by exchanging 80-90% total plasma volume per session (approx 1.5 lts). Replacement of the removed plasma was done with 40% colloids (20% albumin) and 60% crystalloids (normal saline). Post plasmapheresis all the patients were given i.v. Immunoglobulin (10 gm/day) for 5 days. Sirolimus could not be offered as all the patients had significant proteinuria. We did not use Rituximab in any of our patients. However, over a mean follow-up period of 2.5 ± 1.52 years, four (19.04%) patients were lost for follow-up and six (28.57%) patients had stable graft function with mean SCr of 1.89 ± 0.82 mg/dl and urinary protein leak of <+1 by dipstick method. Eleven (52.38%) patients developed graft failure over a mean period of 2.2 ± 1.7 years.
Discussion
CG is now a well-recognized distinct morphological pattern of proliferative parenchymal injury with a poor response to empirical therapy.[7] CG in renal allografts has been reported in literature in the form of few case reports and small studies [Table 5].[891011] In the present study, the overall prevalence of CG in renal transplant was 0.83%. However, we report a higher prevalence of 1.4% in the last 4 years, which shows an increasing awareness of disease in recent years. Similarly, Meehan et al. have also reported an overall prevalence of de novo CG of 0.6%. However, the prevalence in the same study over the period of 1993–1997 was 3.2%.[9]

The predominant native kidney disease was CKD of unknown etiology (57.14%) leading to end stage renal disease, so CG could be de novo/recurrent. The majority of patients presented as end stage renal disease, so we did not have biopsy-proven evidence of native disease of FSGS.
CG was diagnosed over a mean period of 1.85 ± 1.91 years posttransplant. Other reports showed that CG in allograft can occur anytime from 6 to 98 months after transplantation.[9101112] The range of proteinuria in our study was 1.3–6.7 g/24 h and SCr was 1.4–4.6 mg/dl. Swaminathan et al. have also reported high-range proteinuria and high SCr levels in patients with CG versus patients with noncollapsing FSGS in kidney transplant.[12]
Meehan et al. have reported the role of CNI induced hyaline arteriosclerosis and ischemic changes in the development of CG.[9] We have also observed associated CNI toxicity in two patients. One patient out of the two had acute TMA, suggesting role of microvascular injury secondary to CNI toxicity. Other authors have also reported similar findings in their studies suggesting the role of ischemia in the pathogenesis of CG.[9111213] Nadasdy et al. have observed zonal distribution of glomerular collapse in three allograft nephrectomies with obliterative vascular changes and also mentioned that CG in transplant is not same as CG in native kidneys, rather it represents pattern of renal injury.[14]
HIV and parvovirus B19 are known to be associated with CG.[15] We observed SV40 antigen positivity in one patient with CG, suggesting that other viral pathogens may be associated in the genesis of CG. Other authors have also observed the role of BKV in FSGS.[1617]
Shah et al. have mentioned APOL1 polymorphism (G1 and G2) as risk factors for the development of FSGS and chronic kidney disease. They observed two renal allograft failures in recipients who received kidneys from deceased donors having APOL1 polymorphism.[18]
Graft dysfunction may be due to associated acute rejection and immune complex glomerulopathy in CG. We observed 9 (45%) cases of CG with rejection, 8 with acute TCR and AMR, and 1 with chronic TCR. Three patients had immune complex deposits, and recurrence of C3 glomerulopathy was observed in 1 case. Other authors also reported similar results.[91011] Plasmapheresis has been attempted to treat / de novo recurrent focal and segmental glomerulosclerosis with little benefit.[1011] Thus, prognosis of CG remains dismal. Most of the studies have reported that patients with CG eventually develop graft failure over a variable time period.[91011] We also have observed that 11 (52.38%) out of 21 patients in our study developed graft failure over a period of 2.2 ± 1.7 years.
As such there is no evidence based therapy for CG for renal allograft patients. Current regimens comprises of high dose steroids and other immunosuprresants, along with plasmapharesis but success of these regimens is limited.[19]
Conclusions
CG usually presents with moderate to marked proteinuria with rapid graft dysfunction leading to graft failure in most of the transplant patients. CG in renal allograft can coexist with viral infection, drug toxicity, rejection and microvascular injury, etc.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nephrotic syndrome, progressive irreversible renal failure, and glomerular “collapse”: A new clinicopathologic entity? Am J Kidney Dis. 1986;7:20-8.
- [Google Scholar]
- Collapsing glomerulopathy: A clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int. 1994;45:1416-24.
- [Google Scholar]
- The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713-23.
- [Google Scholar]
- KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2010;77:299-311.
- [Google Scholar]
- De novo collapsing glomerulopathy in a renal allograft recipient. Saudi J Kidney Dis Transpl. 2008;19:793-5.
- [Google Scholar]
- De novo collapsing glomerulopathy in renal allografts. Transplantation. 1998;65:1192-7.
- [Google Scholar]
- Collapsing glomerulopathy in renal allograft biopsies: A study of nine cases. Indian J Nephrol. 2011;21:10-3.
- [Google Scholar]
- Collapsing glomerulopathy in renal allografts: A morphological pattern with diverse clinicopathologic associations. Am J Kidney Dis. 1999;33:658-66.
- [Google Scholar]
- Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants. Nephrol Dial Transplant. 2006;21:2607-14.
- [Google Scholar]
- Collapsing focal segmental glomerulosclerosis: Current concepts. World J Nephrol. 2012;1:35-42.
- [Google Scholar]
- Zonal distribution of glomerular collapse in renal allografts: Possible role of vascular changes. Hum Pathol. 2002;33:437-41.
- [Google Scholar]
- Parvovirus B19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am J Kidney Dis. 2000;35:1166-74.
- [Google Scholar]
- Molecular identification of SV40 infection in human subjects and possible association with kidney disease. J Am Soc Nephrol. 2002;13:2320-30.
- [Google Scholar]
- Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488-96.
- [Google Scholar]
- APOL1 polymorphisms in a deceased donor and early presentation of collapsing glomerulopathy and focal segmental glomerulosclerosis in two recipients. Am J Transplant. 2016;16:1923-7.
- [Google Scholar]
- Collapsing glomerulopathy in transplanted kidneys: Only a tip of the iceberg? J Transplant Technol Res. 2011;1:105e.
- [Google Scholar]







