Translate this page into:
Allopurinol-induced Drug Reactions with Eosinophilia and Systemic Symptoms Syndrome with Interstitial Nephritis
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Allopurinol-induced drug reactions with eosinophilia and systemic symptoms (DRESS) is a severe illness related to hypersensitivity syndrome characterized by fever, skin rash, lymph node enlargement, hematological abnormalities, especially eosinophilia and atypical lymphocytosis, and single or multiple organ involvement. The syndrome is difficult to diagnose in view of its clinical heterogeneity and long latency period within 8 weeks after start treatment. We report a case of DRESS syndrome in a 64-year-old man, induced by allopurinol treatment for asymptomatic hyperuricemia, started 8 weeks earlier but stopped only 3 days after because of the onset of rash. The diagnosis was retained due to combining of interstitial nephritis with the clinical findings of fever, skin rash, cervical lymphadenopathy, eosinophilia, and reactivation of human herpesviruses specifically HHV-6. The glucocorticoids were started to relieve hypersensitivity. Five days later, the patient became afebrile, and the rash improved significantly. However, interstitial nephritis with renal function impairment progressed to severe azotemia, and even anuria requiring hemodialysis. Allopurinol-induced DRESS syndrome is associated with significant mortality, and care must, therefore, be exercised when given this drug.
Keywords
Allopurinol
drug reactions with eosinophilia and systemic symptoms
human herpesvirus 6
hypersensitivity
interstitial nephritis
Introduction
Hyperuricemia is a frequent finding and has been related to renal calculi, uric acid (UA) nephropathy, and gout.[1] Allopurinol, a purine inhibitor of the enzyme xanthine oxidase (XO), inhibits the synthesis of UA and has being commonly used drug for hyperuricemia. By inhibiting XO, allopurinol and its primary metabolite alloxanthine (oxypurinol) prevent the conversion of hypoxanthine to xanthine and UA. It provides symptom resolution in most cases with gout; 53% of patients receiving allopurinol 300 mg daily achieve optimal plasma urate concentrations.[2] In some patients, however, it is poorly tolerated and occasionally leads to a severe hypersensitivity reaction. We report a case of drug reaction with eosinophilia and systemic symptoms (DRESS) caused by allopurinol with interstitial nephritis that required intermittent hemodialysis. Despite rapid discontinuation of allopurinol, adequate hydration, and glucocorticoid prescriptions, impaired renal function was irreversible.
Case Report
A 64-year-old man presented with a 2 weeks’ history of fever, pruritus and skin rash, which first appeared on the trunk and then spread to the extremities within 1 week. He complained of shortness of breath with mild, nonproductive cough. He did not present any weight loss, night sweats, headache, abdominal pain, or chest pain. He had a history of 15 years type II diabetes, hypertension and chronic kidney disease (CKD) (baseline creatinine 135 μmol/l; Modification of Diet in Renal Disease clearance of creatinine 46 ml/min/1.73 m2: G3a stage of CKD). The patient was an active smoker (5 pack-years) and had no history of alcohol or illicit drug use. His medications included losartan 50 mg per day, aspirin 75 mg per day and insulin 22 IU morning and 12 IU afternoon for 7 years. Allopurinol for asymptomatic hyperuricemia, 100 mg/day was started 8 weeks previously. On physical examination, his weight was 65 kg, blood pressure 140/60 mmHg, heart rate 88 beats/min, respiratory rate 18 breaths/min, and temperature 38.5°C. The skin lesions were on the trunk and extremities as erythematous macules, slightly involved the palms, spared the scalp, and became more confluent and purpuric over the lower extremities [Figures 1-3]. There were bilateral crackles in both lower lung fields and cervical lymphadenopathy. The liver was palpable 1 cm below the costal margin, and the spleen was impalpable. Examination of heart, rectum, and genitalia was normal. No edema was found on the extremities. His urine output was 600 ml/day. The remainder of examination was normal. Laboratory data included white blood cell count of 8.5 (4.0–10.0 × 109/l) with a high eosinophil count 2.1 (0–0.5 × 109/l). The fasting blood glucose level was 2.1 g/l and HbA1C 9.6%. The alanine aminotransferase 40 (0–40 IU/l) and aspartate aminotransferase 16 (0–40 IU/l). The renal function testing showed significant abnormalities with a raised blood urea nitrogen 78 (2.5–7.5 mmol/l) and creatinine 1196 (50–110 μmol/l). Urinalysis showed mild proteinuria (1 g/day) and leukocyturia without hematuria. Chest radiography showed bilateral interstitial infiltrates. Renal sonography revealed normal renal size without hydronephrosis. The culture of blood and urine was sterile 3 days after hospitalization. Antibiotics were started for the initial impression for pulmonary infection: ceftriaxone 2 g intravenous once daily and ciprofloxacin 200 mg intravenous once daily, and hydration therapy was prescribed. The PCR tests for human herpesvirus 6 (HHV6), Epstein–Barr virus (EBV), and cytomegalovirus (CMV) were positive 2 weeks after hospitalization. Viral hepatitis panel (hepatitis B and hepatitis C) was negative. The skin biopsy specimen taken from a papular lesion on the limb revealed a leukocytoclastic vasculitis. Kidney biopsy was performed 10 days after admission and consistent with interstitial nephritis and signs of diabetic glomerulosclerosis [Figures 4-6]. Combining this interstitial nephritis with the clinical findings of fever, skin rash, cervical lymphadenopathy, eosinophilia, reactivation of HHV specifically HHV-6, the diagnosis of DRESS syndrome caused by allopurinol was made. Oral glucocorticoids (prednisone 1 mg/kg per day) were started to relieve hypersensitivity. Five days later, the patient became afebrile, the cough resolved, and the rash improved significantly. The eosinophil counts also returned to normal value after 3 weeks. However, despite hydration and prednisone soon after admission, renal function impairment progressed with severe azotemia and even anuria where the patient was hospitalized. The patient was then started on intermittent hemodialysis. Prednisone was tapered and stopped 6 months after symptom onset.
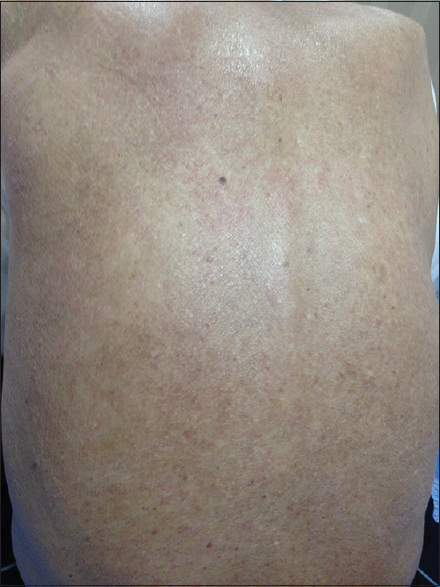
- Erythematous macular lesions over the back

- Erythematous macular lesions over the lower the extremities

- Erythematous macular lesions over the upper extremities

- Dense, diffuse mononuclear interstitial infiltration, periglomerular, perivascular, and peritubular (Periodic acid–Schiff coloration × 40)

- Periglomerular mononuclear interstitial infiltration (Periodic acid–Schiff coloration × 100)

- Dense peritubular, mononuclear interstitial infiltration (Periodic acid–Schiff coloration × 100)
Discussion
DRESS syndrome, previously known as a drug-induced hypersensitivity syndrome (DIHS), is characterized by fever, skin rash, lymph node enlargement, hematological abnormalities, especially eosinophilia and atypical lymphocytosis, and single or multiple organ involvement, which starts within 8 weeks after the start treatment with the offending drug.[34] Diagnostic criteria for DRESS syndrome have been defined in Table 1.[5] Few drugs are known to cause DRESS syndrome. The main drugs are allopurinol, anticonvulsants (phenobarbital, carbamazepine, phenytoin, lamotrigine, and sodium valproate), minocycline, sulfasalazine, disulone, fluindione, proton pump inhibitors, and strontium ranelate.[6] DRESS syndrome is rare (1/5000–10000 prescriptions of each of the causal drugs).[6] The frequency of allopurinol-induced DRESS syndrome is about 1 in 260 patients treated with this drug.[7] Our patient was diagnosed with typical DRESS syndrome based on cutaneous eruptions, fever, lymphadenopathy, eosinophilia, interstitial nephritis, and HHV-6 reactivation developing 8 weeks after starting allopurinol. Diabetes may be a favoring factor of this side effect in our case. The most typical features of DRESS syndrome are related to the timing of the manifestations. There is a long interval of 2 weeks to 2 months from initial drug exposure to symptom onset. Furthermore, the symptoms persist for more than 2 weeks.[6] This was the case in our patient. The most common differential diagnoses of DRESS syndrome include Stevens-Johnson syndrome, toxic epidermal necrolysis, hypereosinophilic syndrome, Kawasaki disease, Still's disease, and viral infections.[7] The genetic susceptibility factor or factors have not yet been identified. It has been associated with specific human leukocyte antigen (HLA) groups in some ethnic groups and for some causal drugs.[8] A study in the Han Chinese population found that HLA-B*5801 is a genetic marker for the DRESS syndrome caused by allopurinol.[9] Although the pathophysiology is still unknown, and two pathophysiology possibilities have been mentioned. The first mechanism links the causal drug to the major histocompatibility complex. The pathophysiology of drug-induced hypersensitivity classically involves a T-cell response induced by the drug or its metabolites after HLA presentation by antigen-presenting cells. The second pathophysiological mechanism involves viral reactivation. Studies done in the past few years have established that the systemic manifestations of DRESS syndrome are related to herpes virus reactivation and to the host immune response against the virus.[6] HHV-6 is a typical example of a latent virus that can undergo reactivation, particularly when immunosuppression occurs.[10] We report a case of allopurinol-induced DRESS syndrome with documented HHV-6, EBV, and CMV reactivation. HHV-6 reactivation is among the criteria developed by Japanese experts for the diagnosis of DRESS syndrome (known in Japan as DIHS).[5] Furthermore, studies established that reactivation of other herpes viruses, namely, the EBV, CMV, and HHV-7, is involved in the systemic manifestations and flares of DRESS syndrome.[111213] Two scenarios have been suggested: an immune response against the drug with secondary viral reactivation related to a cytokine storm, and early viral reactivation responsible for most of the manifestations of DRESS syndrome.[6] The pathophysiology of allopurinol-induced DRESS syndrome seems to be related to the accumulation of oxypurinol in patients with renal insufficiency.[7] In our patient, renal insufficiency may be a contributing factor to developing this syndrome. Studies done by Hande and al.[14] showed that the renal clearance of oxypurinol is directly proportional to the renal clearance of creatinine. There is essentially no renal clearance of oxypurinol when the creatinine clearance has fallen below 10 ml/mn. Avoidance of allopurinol or use of reducing doses in patients with renal insufficiency should be adequate in most patients to avoid hyperuricemia and to reduce allopurinol toxicity.[14] Unfortunately, 80% of patients with allopurinol-induced DRESS syndrome were treated for asymptomatic hyperuricemia.[15] This was the case in our patient. Currently, the only indications for allopurinol include symptomatic hyperuricemia, such as nephrolithiasis and gout, and prophylaxis for urate nephropathy with chemotherapy in neoplastic disease. The allopurinol-induced DRESS syndrome is associated with significant mortality.[7] Accepted treatment consists of early recognition, withdrawal of the drug, and appropriate supportive therapy.[1516] There is controversy regarding the possible beneficial effects of corticosteroids in this disorder.[16] Other methods have been tried in the treatment of DRESS syndrome. Treatment with high dose intravenous N-acetyl cysteine and desensitization in patients with a history of a hypersensitivity reaction to allopurinol requiring additional treatment with this drug have been described.[716]

Conclusion
Allopurinol-induced DRESS syndrome is characterized by cutaneous drug eruptions, eosinophilia and systemic symptoms (lymphadenopathy, hepatitis, interstitial nephritis, interstitial pneumonitis, and/or carditis) that associated with significant mortality. The use of allopurinol for accepted indications and dose adjustment for renal dysfunction are the only ways to decrease the incidence of the potentially fatal toxic effects of this medication.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Disorders of purine and pyrimidine metabolism. In: Brauwald E, Fauci AS, Kasper DC, Hauser SL, Longo DL, Jameson JL, eds. Harrison's Principles of Internal Medicine Vol 2. (15th ed). New York: McGraw-Hill; 2005. p. :2268-73.
- [Google Scholar]
- Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis. 1998;57:545-9.
- [Google Scholar]
- Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug rash with eosinophilia and systemic symptoms: DRESS) Semin Cutan Med Surg. 1996;15:250-7.
- [Google Scholar]
- Drug reactions. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Philadelphia, PA: Elsevier; 2008. p. :301-20.
- [Google Scholar]
- The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007;156:1083-4.
- [Google Scholar]
- HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134-9.
- [Google Scholar]
- HHV-6A, 6B, and 7: Immunobiology and host reponse. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, eds. Human Herpesvirus: Biology, Therapy, and Immunoprophylaxis. Ch. 48. Cambridge: Cambridge University Press; 2007.
- [Google Scholar]
- Association of human herpesvirus 6 reactivation with the flaring and severity of drug-induced hypersensitivity syndrome. Br J Dermatol. 2007;157:934-40.
- [Google Scholar]
- Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol. 2006;155:301-6.
- [Google Scholar]
- Drug-induced hypersensitivity syndrome associated with Epstein-Barr virus infection. Br J Dermatol. 2003;148:1032-4.
- [Google Scholar]
- Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47-56.
- [Google Scholar]
- Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): An update. Dermatology. 2003;206:353-6.
- [Google Scholar]







