Translate this page into:
Metformin-associated Encephalopathy in Hemodialysis
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Metformin-associated encephalopathy in maintenance hemodialysis is very rare in literature, till now only three to four cases are published. We report a patient on maintenance hemodialysis from standalone unit presented to us with abnormal neurological signs and symptoms. His medication chart included metformin, which he was taking for quite a long time. Computed tomography brain showed hypointensity in bilateral basal ganglia. Magnetic resonance imaging (MRI) brain showed hyperintensity in T2/fluid-attenuated inversion recovery sequences suggestive of Lentiform fork sign. We stopped metformin, and he was continued on regular hemodialysis. He showed dramatic improvement in neurological manifestations. Two months later, we repeated MRI brain, which showed resolution of basal ganglia changes. We should suspect the possibility of this when a diabetic end-stage renal disease presents with unknown etiology of encephalopathy.
Keywords
Encephalopathy
end-stage renal disease
Lentiform fork sign
metformin
Introduction
Glucose lowering biguanides were discovered in the 1920s. Due to the association of phenformin and buformin with lactic acidosis, they were withdrawn from the market, but metformin was continued to be used, and in fact, it is considered as the first-line drug of choice for type 2 diabetes mellitus (DM) especially in those with normal renal function. It is contraindicated in those with renal failure, liver disease because it increases the risk of lactic acidosis. It can also rarely induce encephalopathy through lactic acidosis, hypoglycemia, renal impairment, and liver failure. There are only few case reports of metformin encephalopathy in end-stage renal disease (ESRD). we report one such case in a patient with end-stage renal failure undergoing maintenance hemodialysis.
Case Report
Our patient, 63-year-old man a case of type 2 DM, hypertension, old cerebrovascular accident with minimal residual weakness and on maintenance hemodialysis for the past 2 years in outside center. He was on twice-weekly hemodialysis and was compliant. He was admitted to our center with a history of decreased speech output, and difficulty walking for 1 month, with progressive worsening of sensorium, tiredness, drowsiness, and weakness for 2 weeks. No symptomatic improvement noticed after dialysis sessions. No history of fever/headache/focal neurological deficits. Last hemodialysis was done 2 days before admission. On examination, he was drowsy and mute, pulse rate 56/min, blood pressure 180/90 mm of Hg, SpO2 99% on room air, afebrile, and anemic. Neurologic evaluation showed mild weakness of right side limbs with asterexis, rigidity of all limbs and bradykinesia.
Blood investigations revealed blood urea nitrogen 19.63 mmol/L, serum creatinine 609 μmol/L, sodium 138 mmol/L, pottassium 4.8 mmol/L, hemoglobin 104 g/L, total WBC count 4.9, differential count N 57, L 35, M6, E2, platelet count 90,000, serum ammonia 25 umol/L, bilirubin 10.26 μmol/L, serum glutamic pyruvic transaminase 0.47 μkat/L, C-reactive protein 10.48 nmol/L, alkaline phosphatase 4.01 μkat/L, viral markers negative, venous blood gas pH 7.28, HCO3 17.9, PCO2 38, and lactate 7.1.
Noncontrast computed tomography brain [Figure 1] revealed hypodensity in bilateral basal ganglia region. Magnetic resonance imaging (MRI) brain showed hyperintensity in bilateral basal ganglia region (lentiform fork sign) [Figure 2]. Review of his medication chart revealed the long-term use of metformin prescribed by general practisoner at HD unit. We stopped it, and he was continued on regular twice-weekly hemodialysis. After few days, he showed improvement in sensorium. We repeated MRI brain after 2 months, which showed resolution of changes [Figure 3]. Now, he is continuing maintenance hemodialysis.
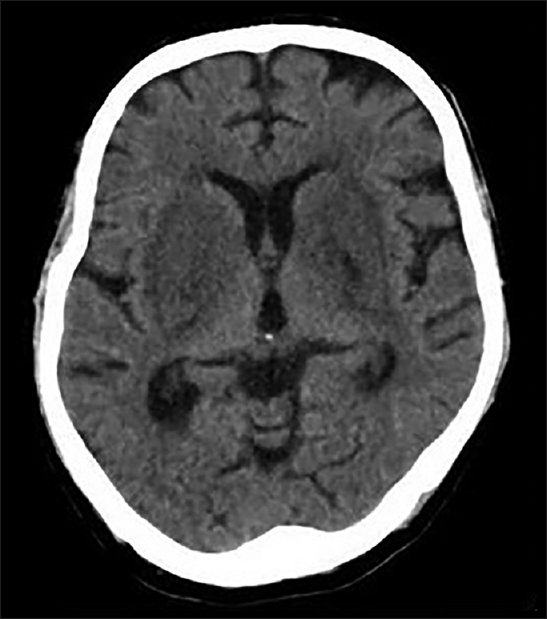
- Noncontrast computed tomography shows hypodensity in bilateral ganglia
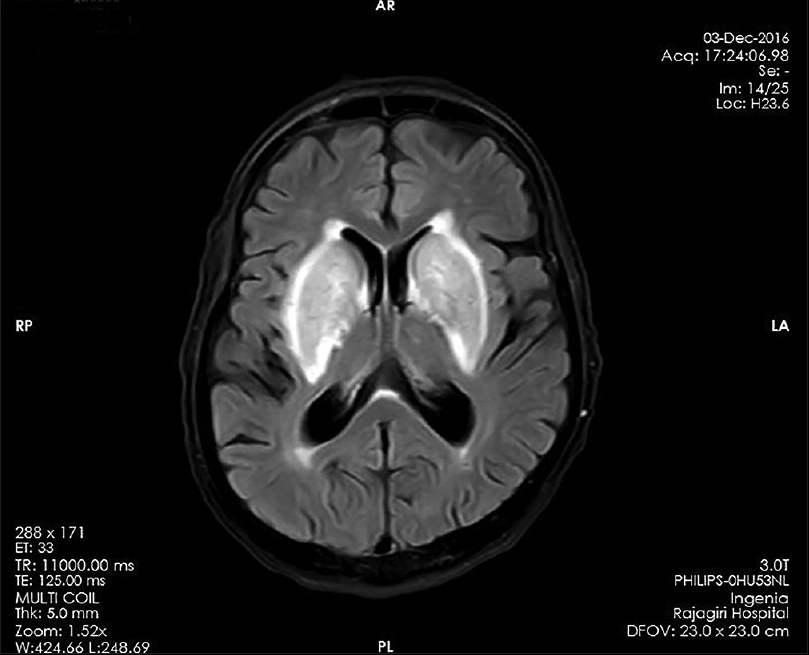
- Magnetic resonance imaging T2/fluid-attenuated inversion recovery with hyperintense basal ganglia

- Magnetic resonance imaging T2/fluid-attenuated inversion recovery with resolution of changes in basal ganglia in repeat imaging 2 months later
Discussion
Even though metformin is a first choice anti-diabetic drug, it is contraindicated in patients with renal failure. Rarely, it can unintentially be prescribed to advanced chronic kidney disease (CKD) population. Holstein et al.[1] reported that 19% of all cases of metformin prescription included patients with renal impairment.
According to the US Food and Drug Administration, metformin should not be used in men and women with serum creatinine concentration >1.5 and >1.4 mg/dl, respectively. However, according to the American Diabetes Association, metformin seems safe unless estimated glomerular filtration rate (eGFR) falls to <30 ml/min/1.73 m2.[23] According to joint position statements of the American Diabetes Association for the study of diabetes agrees that it is reasonable to use metformin down to a GFR of 30 ml/min/1.73 m2 with dose reduction to eGFR 45 ml/min/1.73 m2.[4] However, even the cutoff of 30 ml/min/1.73 m2 GFR is arbitrary, and Mani has reported its use in >1000 poor patients in India with CKD stages 3 and 4 with no case of lactic acidosis.[5]
Exact mechanism of metformin-associated encephalopathy is not clear. It may be caused by overdosage, failure of drug elimination or idiosyncratic susceptibility. Metformin is excreted unchanged in urine, the plasma half-life of metformin may be prolonged, and the drug may easily accumulate in the brain in patients with CKD and ESRD. The progressive neurological manifestations might be explained by cumulative doses resulting from repetitive medications in spite of partial removal by regular dialysis.[6]
The Lentiform fork sign
On T2/fluid-attenuated inversion recovery (FLAIR) sequences, a brightly hyperintense rim delineated the lateral and medial boundaries of both putamina, resembling a fork. The lateral arm of the fork constituted by the edematous external capsule, extended from the anterior end of the putamen at the rostral end of the frontal horn of the lateral ventricle to the stem of the fork, which is formed by the fusion of edematous external and internal capsule at the inferoposterior end of the putamen. The medial arm extended from the stem anteriorly up to one-third of the medial edge where it split into two slightly less T2/FLAIR hyperintense branches engulfing the globus pallidus. These two branches of the medial arm are constituted by the edematous medullary lamina, which divide the lentiform nucleus into three masses (putamen, globus pallidus interna, and externa). Corresponding hypointense changes in T1 sequences [Figure 4].[7]

- Lentiform fork sign
Lentiform fork sign is nonspecific and nondiagnostic, and it has wide differential diagnosis which includes uremic encephalopathy with metabolic acidosis, owing to deranged neurotransmission and cellular metabolism leading to vasogenic edema.[7] Furthermore, methanol intoxication and ethelene glycol toxicity are also associated with this sign. Diabetic uremic encephalopathy also associated with sign may be by the contribution of DKA. The pathogenic basis of this sign may be attributable to the differences in metabolic vulnerability between neurons and astrocytes. As a final common pathway, through changes in vascular reactivity metabolic acidosis may disrupt blood-brain barrier leading to vasogenic edema and later cytotoxic edema depending on the severity of acidosis and other comorbid metabolic perturbations.[7] The putamens high metabolic demand and its location in the boundary zone of vascular perfusion make it particularly susceptible to vascular damage.
In ESRD patients, it can be associated with uremic encephalopathy, but in most cases, it is associated with metabolic acidosis. In our case, there was no severe acidosis and the sign resolved with discontinuation of metformin within 2 months. There is no diagnostic criteria for metformin-associated encephalopathy. Serum safety range is not determined, and also serum level does not reflect tissue level. As in literature,[6] diagnosis can be made with reversal of MRI findings on withdrawal of metformin. There are even case reports of metformin encephalopathy without severe metabolic or lactic acidosis.[8]
Rathi and Mudrabettu[9] published a case report from India in nondiabetic ESRD patient with severe deranged renal function and metabolic acidosis, whose MRI brain showed Lentiform fork sign, but the patient succumbed. In our knowledge, this is the only case from India in diabetic ESRD on metformin presenting with this sign.
Conclusion
Metformin-associated encephalopathy is a rare cause for encephalopathy. Even though metformin is contraindicated in ESRD, patients are unintentially prescribed and on it. It should be suspected in diabetic ESRD patients, especially if no obvious etiology for encephalopathy is found.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Contra-indications to metformin therapy are largely disregarded. Diabet Med. 1999;16:692-6.
- [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11-61.
- [Google Scholar]
- Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
- [Google Scholar]
- Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364-79.
- [Google Scholar]
- Metformin in renal failure – Weigh the evidence. Nephrol Dial Transplant. 2009;24:2287-8.
- [Google Scholar]
- Two additional cases of metformin-associated encephalopathy in patients with end-stage renal disease undergoing hemodialysis. Hemodial Int. 2013;17:111-5.
- [Google Scholar]
- Lentiform fork sign: A unique MRI picture. Is metabolic acidosis responsible? Clin Neurol Neurosurg. 2010;112:805-12.
- [Google Scholar]
- Metformin-induced encephalopathy without lactic acidosis. Diabet Med. 2004;21:194-5.
- [Google Scholar]







