Translate this page into:
Acute cortical necrosis following renal transplantation in a case of sickle cell trait
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Renal transplant recipients who have sickle cell disease are at risk of infection, recurrent graft disease, and sickling crisis that affects the long-term outcome. We report a patient of sickle cell trait who developed patchy cortical necrosis in the perioperative period but had a good long-term outcome. The renal cortical necrosis was presumed to be secondary to cyclosporine-basiliximab interaction in the backdrop of sickling trait. The patient additionally had spontaneous closure of vascular access and severe hypertension immediately following transplantation suggestive of vaso-occlusive crisis. Cyclosporine and basiliximab drug interaction needs to be recognized and steps need to be taken in patients to avoid perioperative graft dysfunction.
Keywords
Immunosuppression
patchy cortical necrosis
renal transplantation
sickle cell trait
Introduction
Renal allograft transplantation has been successful in chronic kidney disease (CKD) stage V patients with sickle cell disease or trait, although incidence of complications has been higher than other causes of CKD-5D.[1] We report a case of sickle cell trait who had developed acute patchy renal cortical necrosis (RCN) following renal transplantation.
Case Report
A 32-year-old woman with sickle cell trait diagnosed on electrophoresis with confirmed sickling phenomenon (on sodium metabisulfite preparation) presented in January 2003 with accelerated hypertension, neuroretinopathy, and CKD stage V. She was initiated on regular hemodialysis. Her pretransplant journey of 1 year was complicated with recurrent pulmonary edema due to breakthrough accelerated hypertension, acute pancreatitis, and tubercular osteomyelitis. Kidney biopsy could not be done to establish the cause of CKD as patient had bilateral small kidneys. The cause appeared to be unrelated to sickle cell trait because of absence of prior episodes of sickling crisis or hematuria and presence of malignant hypertension, all uncommon features in sickle cell trait. She underwent renal transplantation from an HLA mismatched 36-year-old live kidney donor. Triple drug immunosuppression with cyclosporine (8 mg/kg), prednisolone (0.5 mg/kg), and azathioprine (2 mg/kg) was used along with induction therapy with basiliximab (20 mg on day 1 and day 4 of renal transplant). Her preoperative serum creatinine was 6.2 mg/dl. There was brisk post-transplant diuresis (10.5 l on first day and 6.5 l on the second day) and serum creatinine normalized by second day (1.1 mg/dl) as expected in a live renal transplant. She was noted to have spontaneous closure of AV fistula (on day 1) with accelerated hypertension which was controlled with nifedipine, clonidine, and atenolol. On day 5, there was a rise in serum creatinine to 2 mg/dl from a base line of 1 mg/dl, which increased rapidly to 3.8 mg/dl by day 7. However, her urine output remained above 3 L /day and blood pressure was normal (140/80 mmHg) on three drugs. There was no graft tenderness. Investigations showed leukocytosis (WBC count >13500/cu.mm) with near normal hematocrit (PCV 36%) and normal liver function tests (total bilirubin, 1 mg/dl; SGOT, 26 u/ml; SGPT, 32 U/l; serum proteins, 6.2 gm/dl; and albumin, 3.5 gm/dl). Urinalysis was unremarkable with insignificant proteinuria (330 mg/d). Ultrasonogram of graft was normal and radionuclide study of graft showed mild reduction of perfusion with prolonged parenchymal transit. Cyclosporine C2 level (fluorescence polarization immunoassay) on day 7 was 1932 μg/l (target, 1700 μg/l). Graft biopsy done on day 10 showed 15 glomeruli (two viable) with necrosed surrounding tubules, patchy inflammatory infiltrates, and no arteritis [Figure 1a and b]. No renal medullary tissue was seen in the biopsy sample. Acute patchy cortical necrosis was diagnosed and possibility of renal graft rejection or thrombotic microangiopathy as possible alternative diagnosis excluded. Cyclosporine was stopped and mycophenolate mofetil (MMF) substituted for azathioprine. The renal function showed an improving trend (s. creatinine, 3 mg/dl). After 4 days, she developed severe pancytopenia (WBC count, 1000/cu.mm; platelet count, 30 000/cu.mm; and Hb, 7.5 gm/dl). MMF was discontinued and supportive therapy for anemia/leucopenia was given with granulocyte colony stimulating factor, antibiotics, and blood transfusion. Later, sirolimus, MMF, and azathioprine were tried one after the other as second agent but were unsuccessful because of recurrence of leucopenia with each of them. Hence, patient was continued only on high dose of prednisolone 40 mg/day till 6 weeks when her serum creatinine stabilized at 2.2 mg/dl. She was restarted on low-dose cyclosporine (2 mg/kg/day) which was well tolerated, serum creatinine remaining stable. She was discharged from hospital after 7 weeks post-transplant on prednisolone 30 mg/day and cyclosporine 50 mg twice daily (2 mg/kg/day) with serum creatinine 2.2 mg/dl and Hb 9.9 gm/dl. At 72 months follow-up, she is doing well (serum creatinine, 1.2 mg/dl; Hb, 11.5 gm/dl; and leukocyte count, 10 200/cu.mm) and is on low-dose immunosuppression (prednisolone, 7.5 mg/day and cyclosporine, 1.5 mg/kg/day).
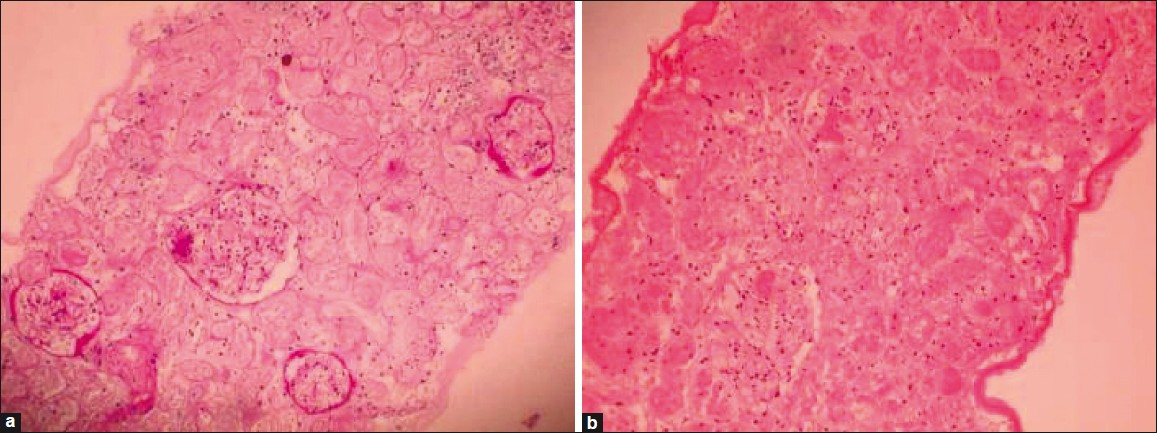
- (a,b) Renal histology (PAS staining, 400× magnifications) showing necrotic areas having ghost outlines of glomeruli and tubules with loss of cellular details
Discussion
Renal manifestations of sickle cell disease are hematuria, renal infarction and papillary necrosis, diminished concentrating ability, renal tubular acidosis, abnormal proximal tubular function, acute renal failure, progressive renal failure with proteinuria (sickle cell nephropathy and less commonly MPGN), and renal medullary carcinoma. Chronic renal disease due to sickle cell trait is very rare,. Chronic sickling is a mechanism of renal injury in sickle cell nephropathy.[2] Relative low oxygen tension of renal arterial system and hypertonic acidic renal medulla combine to promote local red blood cell sequestration, leading to progressive renal ischemia/infarction and interstitial fibrosis.[3] Higher incidence of infection, disease recurrence, and sickling crisis due to improved hematocrit and blood viscosity have contributed to relatively poor long-term clinical outcome in these patients as compared with other etiologies of renal failure after renal transplantation.[4] Such phenomena are mostly seen in sickle cell disease rather than sickle cell trait. Though 1 year patient and allograft survival rates are similar to that of other categories of renal transplantation, 3 year graft survival rate is reported to be significantly inferior. Despite these reported adverse outcomes, long-term survival after renal transplant is still superior to hemodialysis (median survival, 35 vs 24 months)[5] in patients of sickle cell disease. In contrast to sickle cell disease, patients of sickle cell trait have usually benign course with sickling crisis not noted except in extremes of metabolic derangement (e.g., severe acidosis). The possibility of alternative diagnosis of basic disease, specifically compound heterozygote of sickle cell disease (Hb SC and sickle cell-beta (+) thalassemia), were excluded in our patient based on serum electrophoresis and absence of target cells in peripheral blood smear. Absence of anemia, microcytosis, and sickling crisis in the follow-up was consistent with sickle cell trait.
Our patient had several crises, like acute pancreatitis in pretransplant period and spontaneous closure of arteriovenous fistula and acute patchy cortical necrosis in immediate post-transplant period.[4] Such complications could be related or primed by her sickle cell trait. Patchy cortical necrosis causing allograft dysfunction is an uncommon complication, more so in sickle cell trait patient. It was related to cyclosporine-basiliximab interaction following renal transplantation, which resulted in high cyclosporine levels. The raised level of cyclosporine was considered to be the primary event rather than related to renal dysfunction because of known drug interaction of cyclosporine with IL-2 receptor blocker basiliximab.[6] Our success in re-introducing low-dose cyclosporine suggests its long-term safety in this setting. Normal graft function even after 72-month follow-up is reassuring despite contrary reports in literature about poor outcome of such histopathological lesion. Though cyclosporine nephrotoxicity leading to acute graft dysfunction is well known, the role of cyclosporine in producing patchy cortical necrosis is not reported. Cyclosporine upregulates angiotensin-2 receptor and calcium responses in human vascular smooth muscle cells, leading to intrarenal vasoconstriction which could theoretically explain ischemic nephrotoxicity like acute cortical necrosis and hypertension,[7] which was evident in our case. Basiliximab which was used as induction therapy potentiates this vasoconstrictive ischemic injury by increasing cyclosporine levels via cytochrome p 450 pathways, especially in first 10 days of post-transplant period.[6] Cyclosporine could be reintroduced cautiously in such patients without adverse effects at later date as was done for this patient with good medium-term outcome, though one would logically avoid it should the patient tolerate MMF or sirolimus. This report underlies the importance of being aware of such severe drug interaction in susceptible patients with renal injury risk.
RCN after kidney transplantation can result from unusual situations, such as technical surgical problems, graft preservation technique, transplantation across ABO incompatible blood groups, positive cytotoxic antibody crossmatch, and any other factor that can generate hyperacute rejection, thrombotic microangiopathy and prothrombotic state (antiphospholipid syndrome), and drugs (tacrolimus, thymoglobulin). Such an early vascular complication (arterial/venous thrombosis) leading to graft loss/dysfunction remains a constant and devastating complication in 2% of transplantation patients and constitutes a small population of overall acute cortical necrosis (5.3%).[89]
We hypothesize the contribution of sickle cell trait as a co-contributor along with cyclosporine-basiliximab for cortical necrosis. This could be the possible first report of such serious association leading to a complication which could be prevented.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Renal transplantation in end-stage sickle cell nephropathy. Transplantation. 1999;67:291-5.
- [Google Scholar]
- Sickle cell disease. In: Schrier RW, ed. Diseases of Kidney and Urinary tract (7th ed). Philadelphia: Lippincott Williams and Wilkins; 2001. p. :2281-300.
- [Google Scholar]
- National study in natural history of renal allografts in sickle cell disease or trait: a second report. Transplant Proc. 1987;19:33-5.
- [Google Scholar]
- Interleukin-2 receptor antibody-induced alterations of ciclosporin dose requirements in paediatric transplant recipients. Lancet. 2000;356:1327-8.
- [Google Scholar]
- Cyclosporine A up-regulates angiotensin II receptors and calcium responses in human vascular smooth muscle cells. Kidney Int. 1999;55:2407-14.
- [Google Scholar]
- Vascular complications after kidney transplantation. In: Kidney Transpalntation: Principles and Practice (6th ed). Philadelphia: Saunders Elsevier; 2008. p. :446-7.
- [Google Scholar]







