Translate this page into:
Retarding the progression of chronic kidney disease with renin angiotensin system blockade
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We assessed the effect of renin angiotensin system blockade (RASB) in chronic kidney disease (CKD) of diverse etiology. Two hundred and sixty-five consecutive CKD patients attending our renal clinic, with estimated glomerular filtration rate (eGFR) of 20-70 ml/min/1.73m2 at baseline and a minimal follow-up of 1 year, were studied retrospectively. We devised a scoring system to quantify RASB, wherein the maximum dose of an agent recommended for control of hypertension was scored as 1. The renal endpoints studied were the rate of change in eGFR (ΔeGFR) and decline of eGFR>50%. The mean age was 48 ± 11.2 years and 69% were male. The mean duration of follow-up was 4 ± 2.7 years. The rate of ΔeGFR was –1.5 ± 5.0 ml/min/1.73 m2 per year in patients who received RASB (N=168) and –6.0 ± 5.4 in those who did not (N=97) (P<0.001). The incidence of decline of eGFR >50% was 11.3% with RASB and 24.7% without (P=0.003). In a subgroup of patients who received RASB, the incidence of decline of eGFR >50% was 17.8% in the low-dose RASB group (N=84, RASB score 0.63 ± 0.38) and 4.8% in the high-dose group (N=84, RASB score 2.5 ± 0.7) (P=0.001). RASB was associated with significantly better renoprotection in CKD of diverse etiology, even in nonproteinuric diseases. This effect appeared to be dose-dependent, with higher supramaximal doses exhibiting better renoprotection than the lower conventional doses. Our results make a strong case for use of aggressive RASB in all CKD patients to postpone end-stage renal disease.
Keywords
Chronic kidney disease
progression of nephropathy
renin angiotensin system blockade
Introduction
India is a large country of more than a billion population. It is estimated that more than 150 per million develop end-stage renal disease (ESRD) per year in India[12] which puts an enormous burden on the health care system of the country. The vast majority of these patients cannot afford renal replacement therapy on reaching ESRD and hence ESRD is equivalent to death in them.[13] Community-based programs for primary prevention of chronic kidney disease (CKD) are nonexistent in India except for a very few individual efforts.[45] Hence the secondary prevention of ESRD remains the primary focus of the efforts of physicians involved in care of CKD patients.
A large body of evidence exists to show that renin angiotensin system blockade (RASB) using angiotensin-converting enzyme inhibitors (ACEI) and/or angiotensin II receptor blockers (ARB) is very effective in retarding progression of CKD in proteinuric diseases such as diabetic nephropathy[6–8] and glomerulonephritis,[69–15] even when the disease is advanced.[1216] However, their efficacy above and beyond the antihypertensive effect in the progression of nonproteinuric CKD is not established. It is well-recognized that the efficacy of RASB varies in individual patients and race as well as gender have an influence on their efficacy.[1718] The efficacy and safety of combination of ARB and ACEI or supramaximal doses (doses above the maximum recommended for control of hypertension) remains a subject of much debate. Supramaximal doses of ACEI or ARB have an additive effect in reducing proteinuria and dual blockade also has similar effect.[19–22] However, the effects of supramaximal doses of ACEI or ARB on renal outcomes have not been tested sufficiently in clinical trials. The effect of combination therapy on progression of renal disease is conflicting. The only major study (COOPERATE),[23] which showed benefit with combination therapy on renal outcome, has recently been retracted by the publisher, due to irregularities found in the conduct of the study. A recently published ONTARGET study[24] reported that combination of ARB and ACEI caused more rapid decline in glomerular filtration rate (GFR) in patients with high cardiovascular risk compared to either of the agents used alone, causing widespread concern over the use of combination therapy. However, the validity of the design and interpretation of results of the ONTARGET study has been questioned for several reasons.[25] A recent meta-analysis showed that combination therapy and monotherapy were associated with a similar rate of decline in GFR.[26]
In our clinic we have been using aggressive RASB for several years in an attempt to retard progression of CKD of diverse etiology to postpone ESRD and we report our experience of the impact of this strategy on the progression of CKD.
Patients and Methods
This is a retrospective analysis of 265 patients with CKD of diverse etiology, followed over a course of more than one year in the outpatient clinic. The approval of the hospital ethics committee was obtained to conduct the study.
Study population
Five hundred and ten consecutive patients of CKD Stage 2 to 5, with impaired renal function and minimum follow-up of 1 year, who attended the renal out-patient clinic between the period January 1, 2009 and June 30, 2009 were screened. Among them 265 patients had an eGFR between 20 and 70 ml/min/1.73 m2 at baseline and were studied. The following patients were excluded from the study: 1) those who received disease-modifying therapy, such as immunosuppression, intervention to revascularize renal arteries or relief of obstruction and 2) those who had acute renal failure (ARF) or were found retrospectively to have had ARF on their first visit to the clinic.
Setting and patient management
Ours is a busy outpatient clinic affiliated to a renal unit managed by six nephrologists in a tertiary referral hospital. The management of the individual CKD patients was at the discretion of the nephrologist in charge of them. All the nephrologists generally shared the enthusiasm for RASB, but only some used aggressive RASB using supramaximal doses of these agents, which enabled us to get different degrees of RASB in our study population. The management of CKD was generally based on the protocol described in Tables 1 and 2.


Method of monitoring and guiding therapy
Patients who resided within 100 km from the clinic were advised to come at periodic intervals to optimise therapy. Patients who resided beyond 100 km from the clinic (81% of the study population) were advised to follow the treatment protocol which was explained and also given in writing, and were advised to send the reports by telephone, fax, email or letter. Blood pressure, serum creatinine, blood urea and serum potassium were monitored once a week and small increments in RASB agent dose were implemented only after assessing these reports. Patients returned to our unit for assessment at periodic intervals. We had an unplanned control group because some patients found it too troublesome to get their tests performed every week or two, and did not follow their side of the protocol. Some of their primary doctors were opposed too strongly to this treatment and told their patients not to follow our advice. Some patients proved consistently unreliable with treatment, and we could not prescribe potentially dangerous drugs long distance. Most of these patients continued to return to our unit for a check-up at periodic interval despite being taken off the protocol, and they served as a control group. The methodology used for communication is described in detail elsewhere.[3]
RASB
We quantified the amount of RASB achieved in patients and devised a scoring system for this study. The amount of an ARB and ACEI was scored as the dose of that agent used in each patient divided by the maximum recommended antihypertensive dose [Table 2]. The total amount of RASB was quantified by adding the score for ACEI and ARB received by the patient. For example if a patient received 80 mg of enalapril and 50 mg of losartan simultaneously, RASB score (RASB score) in that patient would be 2.5 (80/40 + 50/100 or 2 + 0.5). We noted any adverse effects attributable to the RAS blocking agents.
Demographic data
Data such as age, gender, weight, body mass index (BMI), the place of residence and cause of CKD were collected at the first visit. The diagnosis of the cause of CKD was based on biopsy in 138 (32%) and in the remaining patients was based on clinical criteria described previously.[27] Laboratory data such as serum creatinine, blood urea, serum albumin, serum potassium were collected at baseline, at approximately 12 months, and at the last visit and were done at our hospital. All the laboratory tests used in the analysis were done at our hospital. The blood pressure (BP) and antihypertensive medications received at baseline and at the last follow-up were noted. Proteinuria was not quantified routinely since 24-hour collection was cumbersome for patients coming from elsewhere, who constituted most of our patients. We categorized patients with urinary protein measurements into proteinuric if urinary protein excretion at baseline was more than 1 gm/day and nonproteinuric if less than 1 gm/day. The duration of follow-up was noted for each patient.
GFR estimation and calculations
The estimated glomerular filtration rate (eGFR) was calculated using the standard six variable Modification of Diet in Renal Disease (MDRD) equation at baseline and last visit.[28] The change in eGFR (ΔeGFR) was defined as the difference between eGFR at the last visit and at the first visit. The rate of change in decline in GFR (rate of ΔeGFR) was calculated by dividing ΔeGFR by the duration of follow-up in years and was expressed as ml/min/1.73 m2 per year.
Renal outcomes
The renal endpoints studied were: Rate of ΔeGFR and reduction in eGFR by >50% from baseline during the course of follow-up.
Statistical analyses
The values were expressed as mean±SD, unless specified otherwise. The categorical variables were compared using χ2 test and continuous variables using Student ‘t’ test. Cox regression analysis was performed to identify the factors influencing the renal outcome of reduction in eGFR by >50%. In addition to age and gender, the independent variables with P-value less than 0.1 on univariate analysis were included in Cox-regression analysis. Statistical analysis was performed using SPSS 17.0 statistical software (Chicago, IL, USA).
Results
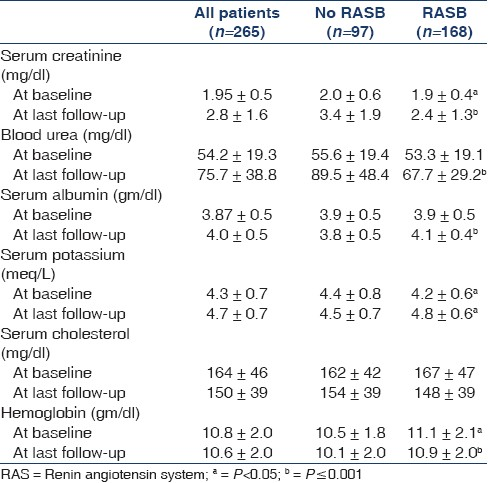
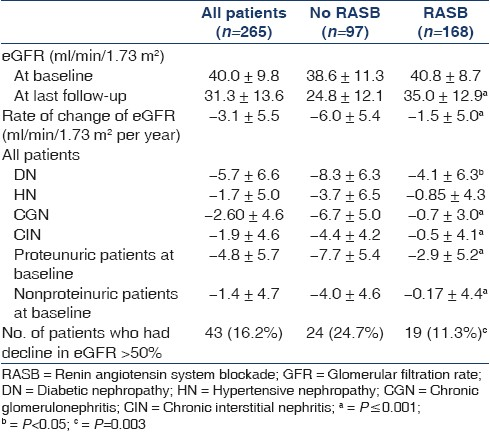
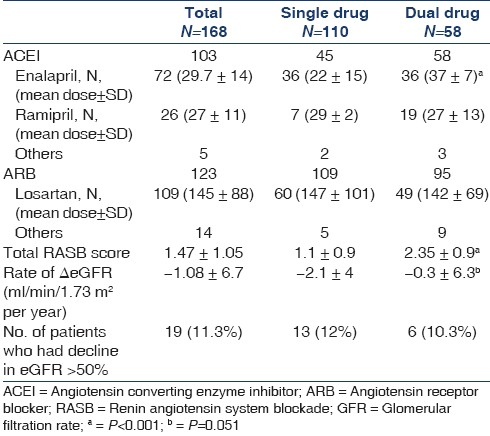
The mean age was 48 ± 11.2 years and 69% were male. The demographic data and laboratory parameters of the patients at base line and at last follow up are shown in Tables 3 and 4, respectively. The eGFR at baseline and at last follow-up and ΔeGFR in different groups of RASB and disease groups are shown in Table 5. The comparison of RASB between single and dual therapy are shown in Table 6. The result of Cox regression analysis to identify variables influencing the renal outcome of decline of eGFR >50% is shown in Table 7.
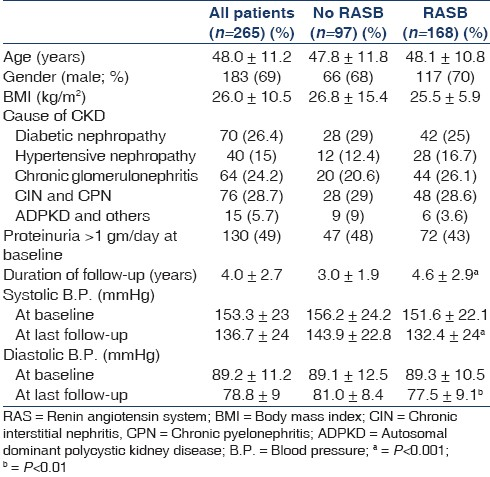

The total number of antihypertensive drugs used was 2.0 ± 1.1 in RASB group and 2.1 ± 1.0 in non-RASB group. Diuretics were used in 37(14%) patients; (15% in RASB and 12% without, P=0.7). The other antihypertensive drugs used were calcium channel blocker in 44% (81% in Non-RASB group, 22% in RASB group, P≤0.001), β-blocker in 28% (47% in Non-RASB and 17% in RAB group, P<0.011), α-blocker in 23% (48% in Non-RASB and 8% in RAS group, P<0.001) and others in 10%. The adverse effects due to drugs were hyperkalemia in 36(13.5%), hypotension in 21(8%), cough in 20(7.5%) and azotemia (increment in blood urea or serum creatinine by >25%) in 27(10%). One hundred and sixty-nine (64%) patients received RASB at baseline and the mean RASB score in them was 0.49 ± 0.3. At last follow-up, 168 (63.4%) received RASB the mean RASB score achieved in them was 1.55 ± 1.1. Supramaximal doses were given in 20% of those who received ACE inhibitors and 48% in those who received ARB. Only 9% of patients had a RASB score of ≥1 at baseline and it increased to 66% at last follow-up. The target systolic blood pressure of ≤130 mmHg was achieved in 132(50%) patients (58% of the RASB group and 36% of the others, P<0.001) and diastolic blood pressure of ≤80 mmHg in 187(70.5%) patients (75% in the RASB group and 63% in others, P=0.001).
A subgroup analysis was done in patients who received RASB (N=168) to assess the effect of degree of RASB on renal outcome in them. Patients were arranged serially from the lowest RASB score to the highest, and divided into two equal groups from the lowest to the median (low dose: N=84, RASB score 0.63 ± 0.38) and from the median to the highest (high dose: N=84, RASB score 2.5 ± 0.7). The duration of follow-up was similar in both groups (4.3 ± 3.2 years in low-dose and 4.8 ± 2.5 years in high-dose group, P=0.28). The rate of ΔeGFR (ml/min/1.73 m2 per year) was –2.03 ± 5.1 in the low-dose and 0.89 ± 4.8 in the high-dose groups (P=0.13). Fifteen patients (17.8%) in the low dose group and 4 (4.8%) in the high-dose group had a decline in eGFR >50% during follow-up (P=0.001).
The relationship between ΔeGFR and time in patients who received RASB and controls and the projected time to reach ESRD is shown in Figure 1. The relationship between the rate of ΔeGFR and degree of RASB (RASB score), including cubic regression line is shown in the Figure 2. Figure 3 shows the interaction between degree of RASB and blood pressure control with regard to renal outcome of decline in eGFR >50%.

- Scatter plot showing the change in GFR over a period of time in RAS blockade and no RAS blockade
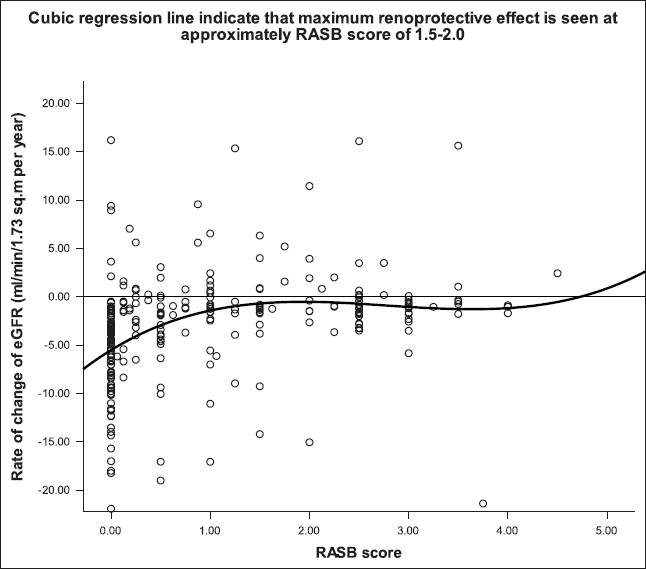
- Scatter plot with cubic regression line, showing the relationship between RASB score and the rate of ΔeGFR
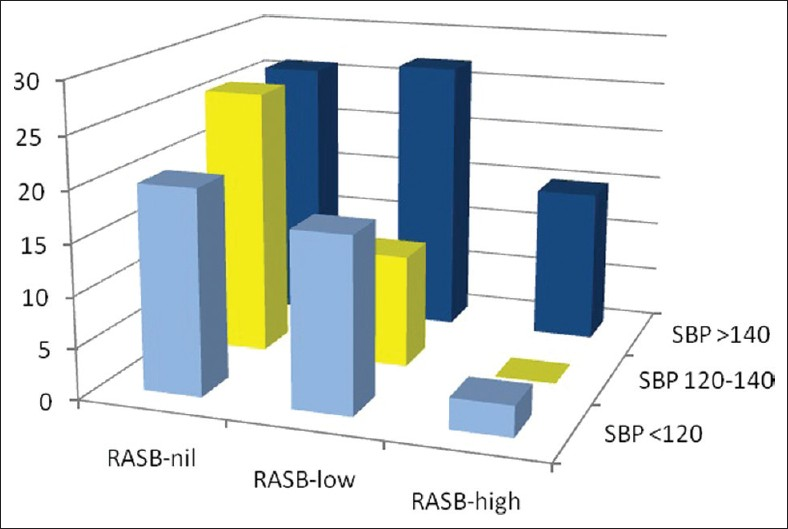
- The graph depicting the interaction between degree of RAS blockade and blood pressure control on renal outcome on decline in eGFR >50% (RASB, rennin angiotensin system blockade; SBP, systolic blood pressure at last follow up; Bars represent incidence in percent, of renal outcome of decline in eGFR >50%)
Discussion
We assessed the impact of varying degrees of RASB in a large population of CKD of diverse etiology. Previous studies have been done with fixed doses of ACEI or ARB. Our results showed that RASB significantly slowed the decline of eGFR in all major diseases causing CKD except hypertensive nephropathy. Patients who received RASB had better preservation of GFR than those who did not and were less likely to have 50% or more decline in eGFR over a mean follow-up of 4 years (24.7% vs 11.3%, P=0.03). The projected time to reach ESRD was also markedly prolonged in patients receiving RASB, compared to the control group [Figure 1]. The independent determinants of the preservation of GFR in our study population were RASB intervention, nonproteinuric state (<1 gm/day) at the baseline, lower systolic blood pressure and higher serum albumin achieved with therapy.
The dose of an ACEI or ARB for maximum renoprotection remains unknown and it is increasingly felt that it is probably much higher than the dose recommended for maximum antihypertensive effect.[29] We quantified RASB using a scoring system developed specifically for this study. We noted that the renoprotection conferred by RASB appeared to be dose-dependent to an extent and the dose required to achieve maximum renoprotection appeared to depend on the cause of CKD and baseline GFR. Higher supramaximal doses (mean RASB score: 2.5 ± 0.7) of RASB were associated with a significantly lower incidence of decline in eGFR of >50% compared to lower submaximal doses (mean RASB score: 0.63 ± 0.38) over a mean period of 4.5 years (17.8% vs 4.8%, P=0.001). Cubic regression analysis [Figure 2] showed that optimal protection was achieved with a RASB score of approximately 1.5 to 2 in all patients, indicating that a supramaximal dose of a single agent or maximal doses of two agents is likely to confer maximal benefit. The RASB score at which maximum benefit was achieved in diabetic nephropathy was 3, in chronic glomerulonephritis was 1.5 and in chronic interstitial nephritis was 1 (not shown in results). These results indicate that more aggressive RASB is beneficial in proteinuric patients, whereas less aggressive blockade is sufficient to confer maximum benefit in nonproteinuric diseases such as chronic interstitial nephritis.
One could suspect that the higher dose of RASB was driven by the more resistant hypertension, thereby enjoying better renoprotection in our study population. This was not the case because the dose of RASB was not primarily driven by the degree of hypertension. Indeed, other antihypertensive agents were also used in the initial period of blood pressure control along with RAS blocking agent and were withdrawn slowly once blood pressure was under reasonable control, thereby facilitating further increment in the dose of RASB agents. The average number of drugs used in two groups was similar. Systolic blood pressure was higher in non-RASB group at the end of last follow-up mainly due to the nonadherence to the protocol, which again reflected noncompliance. When analyzing our data we were aware of the two confounding factors namely blood pressure control and RASB on the renal outcome. To tease out the individual contribution of these two confounding factors, we performed Cox regression analysis which showed that the RASB but not the systolic blood pressure achieved was associated with better outcome [Table 7]. Moreover, we wanted to analyze this further and hence looked at the interaction of systolic blood pressure and dose of RASB at different levels of these two parameters, which is shown in Figure 3. These analyses clearly show that RASB has a renal protective effect over and above the blood pressure control effect.
We would like to draw comparison between our study and a recent study by Ruggenenti et al. (Remission Clinic study).[30] There are many similarities between them: a) multimodal approach to CKD management, b) similar duration of follow-up, c) retrospective analysis of CKD patients followed in the outpatient clinic and d) RASB using single or two agents. However, our study differed in a few ways: a) the number of patients in our study was larger, b) we targeted higher doses of RASB than the Remission Clinic study and c) we included nonproteinuric patients for aggressive RASB, whereas only proteinuric patients were included in the other study. Ruggenenti et al. using a multimodal regimen titrated to urinary protein excretion, achieved disease remission or regression in up to 50% of patients who had severe CKD and were otherwise expected to progress rapidly to ESRD while on conventional therapy titrated just to blood pressure control. They achieved a rate of decline of GFR of –2.04 ml/min/1.73 m2 per year in their intervention group. We had an overall rate of decline in eGFR of –1.5 ± 5.0 ml/min/1.73 m2 per year in patients who received RASB. The rate of decline in eGFR was –6.7 ml/min/1.73 m2 per year in their control population, whereas it was -6.0 ± 5.4 ml/min/1.73 m2 per year in our control patients who did not receive RASB. A large randomized multicentre (ONTARGET) study published recently showed that combination therapy with ACEI and ARB was associated with more rapid decline in GFR compared to a single agent in predominantly elderly, nonproteinuric patients with cardiovascular disease and in high-risk diabetes CKD patients.[24] Our study population differed markedly from that of the ONTARGET study in that our patients were younger and were not considered at high risk for cardiovascular disease. We feel that generalizing the results of ONTARGET study to all CKD patients is not only incorrect, but dangerous. The vast majority of CKD patients encountered in our clinic do not fall within that described in the ONTARGET study. The rate of decline of eGFR in dual therapy in our study population was comparable to that in single-agent therapy (-0.3 ± 6.3 vs -2.1 ± 4, P=0.051). The other renal outcome of decline in GFR >50% in dual and single agent therapy was similar (10.3% vs 12%, P=NS). Similar findings were reported in a recent meta-analysis wherein combination therapy compared to monotherapy had a similar rate of decline in GFR.[26] Our results indicate that the higher dose rather than combination therapy confers better renoprotection in CKD.
The concerns about safety with dual blockade or supramaximal doses of drugs are based more on speculation than on evidence. The safety of RASB has been shown in several trials including in patients with advanced CKD with GFR of <30 ml/min.[1516] Meta-analysis of dual blockade therapy has shown that very few patients developed drug-related complications requiring withdrawal and there were no deaths.[2126] Burgess et al. used doses up to eight times higher than the maximum recommended doses of candesartan and found no significant increase in serious side effects and reported no death.[18] The RASB in our patients was generally well-tolerated. However, RASB is not free of side effects. The adverse effects noted in our study limiting further increment in the dose of RASB were hyperkalemia in 13.5%, cough in 7.5%, increment in azotemia in 10% and hypotension in 8%. Since the therapy was closely monitored and increments in doses were done slowly in stepwise fashion and corrective measures were taken when complications occurred, we did not encounter a life-threatening situation attributable to therapy in any of our study population. Our study showed that therapy to achieve optimal RASB is effective and safe even when patients were monitored from a distance by a modern communication system. We could not have achieved much success in our endeavor if not for the revolution in communications currently sweeping third world countries. Based on our experience, we feel that modern systems of communication have an important role in managing CKD in developing countries.
There are several strengths to our study. First, we have shown, to our knowledge for the first time, a significantly better renoprotective effect of supramaximal doses of RASB compared to lower conventional doses of RASB in CKD of diverse etiology. Second, we have shown a renoprotective effect in predominantly nonproteinuric disease such as chronic interstitial nephritis, which has not previously been reported, mainly because it has not been sufficiently studied. Chronic interstitial nephritis is more common in India than elsewhere,[27] and this enabled us to test the efficacy of RASB in patients with this condition who constituted a large number of our study population. Third, we have documented an effective innovative method of monitoring and managing RASB therapy using modern communication systems in patients residing far away, which has a major implication for clinical practice in developing countries. Fourth, we have shown the efficacy of RASB in retarding the progression of CKD in a large south Asian population, which has not been documented previously. This is important in the view of the reported racial differences in organ protection conferred by RASB.[18]
There are several limitations to our study. First, all the limitations of a retrospective analysis apply to our study. The study groups were not preplanned and intervention was not randomized. However, due to heterogeneity in the application of RASB between different nephrologists in our unit and varying compliance with therapy, we have achieved a large control group and different gradations of RASB, which allowed us to draw meaningful conclusions. Second, proteinuria was not quantified and hence we could not ascertain the exact impact of different degrees of RASB on proteinuria, which might have influenced the progression of CKD. Instead, we used the change in the serum albumin following intervention as a surrogate for the control of proteinuria, since studies have shown a good correlation between serum albumin and proteinuria in proteinuric CKD population.[3132] This improved significantly in patients who received RASB and did not change in others. Third, the impact of other factors such as smoking and adherence to prescribed low protein diet was not assessed in our study. However, we feel that their impact is minimal at best since active smoking is less common (<10%) in our CKD population and protein intake is generally very low in Indians even before a dietary intervention is made.[33] Fourth, the scoring system to quantify RASB was evolved for this study and has not been described before. It was based on the assumption that maximum doses recommended for control of hypertension are also equivalent for renoprotection between ACE inhibitors and ARB, which remains to be proven. However, the quantification of antihypertensive drugs in studies related to hypertension control has been published and we followed the same principle in our study to devise the scoring system.[34]
Conclusions
Our study showed that with careful monitoring, increasing RASB to the maximum tolerated using multiple agents in supramaximal doses can be achieved safely in the majority of CKD patients. Such an intervention was associated with significantly better renoprotection in CKD patients of diverse etiology including nonproteinuric diseases and the effect appeared to be dose dependent. Our results make a strong case for use of aggressive RASB in all CKD patients to postpone ESRD.
Source of Support: Nil
Conflict of Interest: None declared.
References
- The incidence of end-stage renal disease in India: A population-based study. Kidney Int. 2006;70:2131-3.
- [Google Scholar]
- Treating renal disease in India's poor - the art of the possible. Semin Nephrol. 2010;30:74-80.
- [Google Scholar]
- Nephrologists sans frontiers: Preventing chronic kidney disease on a shoestring. Kidney Int. 2006;70:821-3.
- [Google Scholar]
- Experience with a programme for prevention of chronic renal failure in India. Kidney Int Suppl. 2005;94:S75-8.
- [Google Scholar]
- Effect of ACE inhibitors in diabetic and nondiabetic chronic renal disease: A systematic overview of randomized placebo-controlled trials. Am J Kidney Dis. 2000;35:695-707.
- [Google Scholar]
- Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-60.
- [Google Scholar]
- Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;20(345):861-9.
- [Google Scholar]
- Angiotensin-converting enzyme inhibition and renal protection in nondiabetic patients: The data of the meta-analyses. J Am Soc Nephrol. 2005;16(Suppl 1):S58-63.
- [Google Scholar]
- The effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: A pooled analysis of individual-patient data from 11 randomized, controlled trials. Ann Intern Med. 2001;135:138-9.
- [Google Scholar]
- Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: A patient-level meta-analysis. Ann Intern Med. 2003;39:244-52.
- [Google Scholar]
- Gruppo Italiano di Studi Epidemiologici in Nefrologia: ACE inhibitors to prevent end-stage renal disease: When to start and why possibly never to stop: A post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol. 2001;12:2832-2837.
- [Google Scholar]
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomized placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857-63.
- [Google Scholar]
- Renal function and requirement for dialysis in chronic nephropathy patients on long-term ramipril: REIN follow-up trial. Gruppo Italiano di Studi Epidemiologici in Nefrologia (GISEN). Ramipril Efficacy in Nephropathy. Lancet. 1998;352:1252-6.
- [Google Scholar]
- Renoprotection of Optimal Antiproteinuric Doses (ROAD) study: A randomized controlled study of benazepril and losartan in chronic renal insufficiency. J Am Soc Nephrol. 2007;18:1889-98.
- [Google Scholar]
- Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354:131-40.
- [Google Scholar]
- Chronic proteinuric nephropathies. II. Outcomes and response to treatment in a prospective cohort of 352 patients: Differences between women and men in relation to the ACE gene polymorphism. Gruppo Italiano di Studi Epidemologici in Nefrologia (GISEN) J Am Soc Nephrol. 2000;11:88-96.
- [Google Scholar]
- Racial differences in the renal response to blood pressure lowering during chronic angiotensin-converting enzyme inhibition: A prospective double-blind randomized comparison of fosinopril and lisinopril in older hypertensive patients with chronic renal insufficiency. Am J Kidney Dis. 1997;29:897-906.
- [Google Scholar]
- Supramaximal dose of candesartan in proteinuric renal disease. J Am Soc Nephrol. 2009;20:893-900.
- [Google Scholar]
- Enhanced renoprotective effects of ultrahigh doses of irbesartan in patients with type-2 diabetes and microalbuminuria. Kidney Int. 2005;68:1190-8.
- [Google Scholar]
- Meta-analysis: Effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148:30-48.
- [Google Scholar]
- Antiproteinuric response to dual blockade of the renin-angiotensin system in primary glomerulonephritis: Meta-analysis and metaregression. Am J Kidney Dis. 2008;52:475-85.
- [Google Scholar]
- Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): A randomized controlled trial. Lancet. 2003;361:117-24.
- [Google Scholar]
- Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomized, double-blind, controlled trial. Lancet. 2008;372:547-53.
- [Google Scholar]
- Dual RAS Therapy not on target, but fully alive. Nephron Clin Pract. 2010;116:c137-42.
- [Google Scholar]
- Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: A systematic review of the efficacy and safety data. Am J Kidney Dis. 2006;48:8-20.
- [Google Scholar]
- Chronic renal failure in India. Nephrol Dial Transplant. 1993;8:648-9. discussion 683
- [Google Scholar]
- A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-70.
- [Google Scholar]
- Drug dosing for renoprotection: Maybe it's time for a drug efficacy-safety score? J Am Soc Nephrol. 2009;20:688-9.
- [Google Scholar]
- Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol. 2008;19:1213-24.
- [Google Scholar]
- Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754-62.
- [Google Scholar]
- Serum albumin levels in different stages of type 2 diabetic nephropathy patients. Indian J Nephrol. 2004;14:89-92.
- [Google Scholar]
- The role of dietary protein restriction in Indian patients with chronic renal failure. J Assoc Physicians India. 2000;48:1078-81.
- [Google Scholar]
- Antihypertensive treatment based on blood pressure measurement at home or in the physician's office: A randomized controlled trial. JAMA. 2004;291:955-64.
- [Google Scholar]
- Anonym. Physicians’ Desk Reference. 2007. (61st ed). Montvale NJ, USA: Thomson PD; Available from: http://(www.PDR.net)
- [Google Scholar]







