Translate this page into:
Renal disease in human immunodeficiency virus — Not just HIV-associated nephropathy
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The aim of the study was to determine the various histopathological lesions in human immunodeficiency virus (HIV) patients with renal dysfunction and to establish clinicopathological correlation. Over a period of two years from January 2008 to March 2010, 27 HIV positive patients with renal dysfunction were subjected to renal biopsy. Of the 27 patients, 23 were males and four were females (85.2% males, 14.8% females). Mean age was 38.2 ± 10.36 (range 20 – 60) years. The probable mode of acquisition of HIV infection was sexual in 22 patients (81.5%). Thirteen patients (48%) had nephrotic proteinuria. The CD4 count ranged from 77 to 633/microliter. The kidneys were of normal size in 19 (70.4%) and bulky in eight (29.6%) patients. Thirteen patients required renal replacement therapy. Eleven patients had acute tubule-interstitial lesions (40.7%) while 15 (55.5%) had glomerular lesions. The various glomerular lesions were, focal segmental glomerulosclerosis in five, amyloidosis in three, diffuse proliferative GN in two, and membranoproliferative glomerulonephritis (GN), membranous GN, minimal change disease, diabetic nephropathy, crescentic GN, and thrombotic microangiopathy were seen in one each. None of the clinical or laboratory variables, except hypertension, was found to predict glomerular versus non-glomerular lesions on biopsy. In conclusion we show that a variety of glomerular and tubulointerstitial lesions can be seen on renal histology. Hence, renal biopsy is indicated in renal dysfunction associated with HIV for making proper diagnosis and therapy.
Keywords
Acute kidney injury
human immunodeficiency virus
HIV-associated nephropathy
renal histology
nephrotic syndrome
Introduction
The human immunodeficiency virus (HIV) infection has become a global pandemic. The size of the HIV-infected population and the longevity of HIV-affected patients is increasing due to the highly active anti-retroviral therapy (HAART). As a result a lot of diseases affecting various organ systems in the normal population are becoming manifest in these HIV patients. One such spectrum of diseases is renal involvement in HIV patients. Previously, only few diseases such as collapsing focal segmental glomerulosclerosis (FSGS) were thought to be prevalent in HIV patients, but now the entire spectrum of renal diseases in an otherwise normal population is being witnessed in the HIV population too. There is paucity of data on the histopathological lesions in HIV from our country.
The study was carried out to determine the various histopathological lesions in HIV patients with renal dysfunction, necessitating renal biopsy, and to establish the clinicopathological correlation.
Materials and Methods
Over a period of two years (January 2008 to March 2010), 4337 patients were registered at our hospital's antiretroviral therapy (ART) center, out of whom 1330 patients received HAART. From the ART center, 63 patients were referred to the Nephrology Department for the evaluation of renal dysfunction. Out of these 63 patients, 36 patients recovered spontaneously and hence were not investigated further. Twenty-seven patients with unexplained renal failure or nephrotic proteinuria underwent renal biopsy. A detailed history and examination was carried out in these patients. The CD4 count was measured at out ART center by flow cytometry. The patients were stratified using the 1993 revised classification system for HIV infection and an expanded surveillance case definition.[1] Biopsy was performed under ultrasound guidance with a biopsy gun (BARD 16/18 G, 22 mm, cutting edge) and subjected to light and immunofluorescence microscopy. Dialytic support was given as needed — either intermittent peritoneal dialysis (IPD) or hemodialysis (HD). Statistical analysis was performed by utilizing the SPSS software.
Results
Of the total 27 cases, 23 were males and four were females (85.2% males, 14.8% females). Mean age was 38.2 ± 10.36 (range 20 – 60) years. Twenty-five patients were married (92.6%). All patients were positive exclusively for the HIV-1 strain.
Fifteen patients were in category I (55.5%), nine in category II (33.3%), and three (11.1%) in category III based on the CDC classification.[1] Eight patients (29.6%) were on HAART at the time of presentation with with renal dysfunction. HAART regimen was lamivudine 150 mg×BD, stavudine 30 mg and efavirenz 600 mg×QD. The indication for the initiation of HAART was acute infection syndrome, chronic infection, and symptomatic disease (including HIV-associated nephropathy (HIVAN) and patients with CD4+ T cell count <200/microliter).
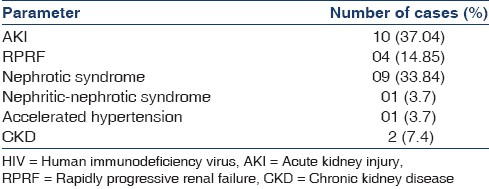
The probable mode of acquisition of HIV infection was sexual in 22 patients (81.5%) and non-sexual in the remaining five (18.5%), that is, by non-sterile needle sharing for therapeutic injections in two patients, blood transfusions in two, and unknown mode in one. The HIV status of the partner was positive in 12/27 patients (44.4%). The average duration of HIV illness by the time the patients presented to the Nephrology Department was 16.5 ± 25.29 months. The maximum duration of HIV infection was nine years. The patients presented with varied clinical syndromes as depicted in Table 1. Many patients had associated comorbidities as shown in the Table 2. Hypertension was seen in 40.7%. The mean SBP was 132 ± 21.29 mm of Hg. Twelve patients were on infectious disease prophylaxis, namely, trimethoprim and sulfamethoxazole.

Seventeen patients had raised serum creatinine. The serum creatinine at the time of presentation ranged between 1.2 and 17 mg/dl (mean 5.7 ± 4.30 mg/dl). The mean blood urea was 92.48 ± 54.48 mg/dl; Mean serum potassium was 4.17 ± 0.65 meq/L; hematocrit ranged between 22 and 42 volume % (mean 28.8 ± 5.57). Neutrophilic leucocytosis was seen in four. None had thrombocytopenia. The microangiopathic blood picture was found in only one patient and this patient was found to have thrombotic microangiopathy. Low C3 was found in two (biopsy showed post infectious glomerulonephritits (PIGN) in these). Thirteen patients (48%) had nephrotic proteinuria. The mean 24 hours urinary protein was 2.6 ± 2.53 g (range 0.4 to 13 g/day). The CD4 count ranged from 77 to 633/microliter. The average CD4 counts in patients with various clinical syndromes were as follows: Acute kidney injury (AKI): 297 cells/microliter, chronic kidney disease (CKD): 283 cells/microliter, nephrotic – nephritic illness: 92/microliter; nephrotic syndrome: 276/microliter, and in rapidly progressive renal failure (RPRF) 298/microliter. The kidneys were normal sized in 19 (70.4%) and bulky in eight patients (29.6%). Thirteen patients required renal replacement therapy (four received hemodialysis and nine patients received intermittent peritoneal dialysis). The histopathological lesions on renal biopsy are shown in the Table 3. The biopsy samples were adequate with an average of nine glomeruli. Crescents were noted in three patients (one had paucimmune crescentic GN and the remaining two had diffuse proliferative GN). Mesangial hypercellularity was noted in 10 patients (37%) with mesangial fibrosis in eight and interstitial inflammation in 23 (85.1%). Eight patients (29.6%) had hyalinization of vessels. Immune deposits were noted in 26 cases — 19 patients had C3, six had C3, IgM and IgG, while one had C3 and IgM deposits. One patient had the rare combination of amyloidosis with diffuse proliferative glomerulonephritis [Figures 1 and 2]. None had interstitial granulomas or segmental necrosis. Chronicity was noted in five patients (18.5%). None of the variables except hypertension was found to predict glomerular versus non-glomerular lesions on biopsy.


- Amyloidosis with diffuse proliferative glomerulonephritis in HIV (H and E, ×40)
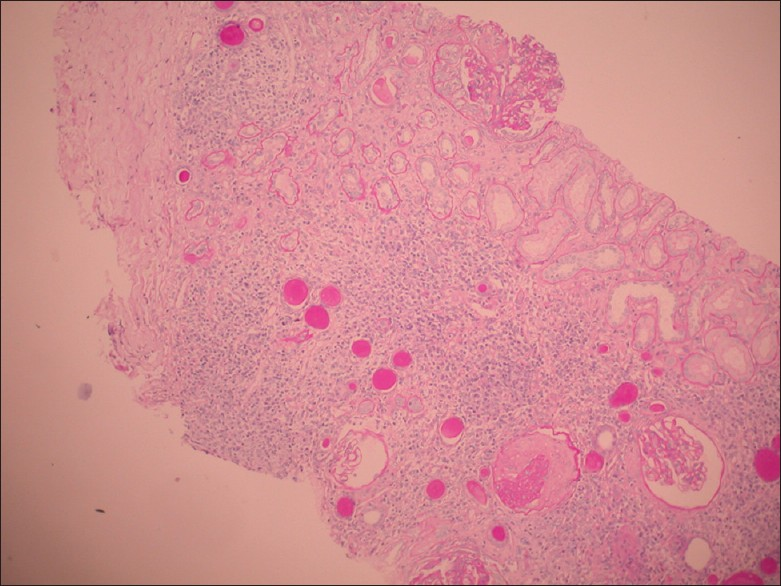
- Amyloidosis with diffuse proliferative glomerulonephritis in HIV (H and E, ×10)
Discussion
Renal disease in patients infected with HIV was first described by Rao et al.[2] in 1984, as a focal and segmental glomerulonephritis subsequently termed as ‘HIVAN’. The prevalence of HIVAN has been reported to be from 3 to 7% and the US Renal data system has reported a steady increase in the incidence of HIVAN.[3] HIVAN is best characterized by nephrotic range proteinuria, azotemia, normal-to-large kidneys on ultrasound (US) scan, normal blood pressure, and collapsing FSGS on renal biopsy.
As the prevalence of HIV is increasing, the spectrum of renal disorders in HIV patients is also changing. HIVAN, which used to be synonymous with HIV renal disease in the first two decades of the HIV pandemic has been replaced with much more common renal disorders, namely, AKI and other glomerular diseases.
Peraldi et al.[4] described 10 different causes of AKI and RPRF in their study, where they studied 91 patients. The different causes of AKI were hemolytic uremic syndrome (HUS) (32), Acute tubular necrosis (ATN) (18), Obstructive renal failure (16), HIVAN (14), Acute interstitial nephritis (AIN) (2), and various glomerulonephritis in four patients. In a study by Mathur et al.,[5] out of 42 HIV cases, six had renal failure, although none underwent renal biopsy. In the present study, 14 out of 27 patients presented with AKI and RPRF (51.8%).
Renal involvement in HIV-positive patients was more common in males in our study. In the study by Peraldi et al.,[4] the male:female ratio was 7:1. In a Nigerian study,[6] the male:female ratio was 1:1.
In a study, HIV-associated renal disease was reported in all the risk groups including heterosexuals, homosexuals, perinatal acquisition, exposure to contaminated blood products, and IV drugs abusers.[7] In an Indian study by Janakiraman et al.,[8] 96% got infected by heterosexual contact, 3% by transfusion, and 1% by IV drug abuse. In the current study, the heterosexual contact was the chief mode of transmission.
Human immunodeficiency virus-associated renal disease can present at any stage of HIV illness with the exception of HIVAN, which occurs late in the course of the illness. In the study by Marie Noelle et al.,[4] the average duration prior to the onset of HIVAN was eight months, 59 months for HUS, and 56 months for ATN. In the current study, the average time period between the diagnosis of HIV infection and the renal dysfunction was 17.1 months for those with glomerular disorders and 15.7 months for those with tubulointerstitial and vascular disorders. The average time to progression to uremia was three to four months in a study by Agati et al.[9] The prevalence of hypertension was low in patients with HIVAN in spite of severe renal dysfunction.[10] However, in the current study, the prevalence of hypertension was 40.7%. In addition to renal dysfunction various comorbidities were seen in HIV patients with renal disease. In the study from France,[4] candidiasis was noted in 42%, Kaposi sarcoma in 25%, CMV infection in 28%, mycobacterial infection in 14%, toxoplasmosis in 13%, and pneumocystis in 7.5%. The probable reason for the apparent absence of pneumocystis infection and toxoplasmosis in the current study could be due to the current policy of giving co-trimoxazole prophylaxis to every patient with a CD4 count <300/microliter.
HIV-associated nephropathy had a significant geographical and ethical diversity, with prevalence being 2% among the HIV-positive nephrotics in San Fransisco, 15.2% in France,[4] and 83% in a study by Bourgoignie et al.[10] In an Indian study, seven out of ten patients had features of HIVAN.[8] In the Columbia Presbyterian Multicenter study,[9] 26 out of 73 patients with HIVAN had proteinuria of >6.6 g/day. In the present study, all the three patients of HIVAN had an average proteinuria of 3.7 g/day. The average kidney size in HIVAN patients in Columbia[9] study was 12.3 cm. However, renomegaly was not a universal finding in our study. HIVAN usually presented in a setting of low CD4 counts. In a study from France,[4] the average CD4 count was 86/mm3 (range: 1 – 600), in patients with HIVAN, – 139/mm3, 43/mm3 in those with HUS, 125 in those with AIN, and 216 in those with ATN. In the current study, the mean CD4 count was 299/microliter in patients with tubulointerstitial or vascular pathology and 265/microliter in those with glomerular lesions. There were few studies in the literature that described the histological changes in HIV-positive patients with renal dysfunction[11–14] [Table 4]. In these studies glomerular disorders constituted 78% while tubulointestitial and vascular disorders constituted 22%. This is in contrast to the present study wherein the glomerular lesions were seen in 55.6% with collapsing FSGS in only 11.2% of glomerular lesions while tubulointerstitial and vascular lesions were seen in 44.5%. As seen in the general population, all the common renal pathological lesions were seen in the study cohort. This may be due to three reasons. First, there may be a change in the pattern of HIV-related renal diseases. The second reason could be due to early screening and treatment. The third reason could be the increasing awareness among the general practitioners and timely referral to the nephrologists.

Emem et al.[6] suggested that age, BMI, serum albumin, and CD4 counts may be risk factors for nephropathy in HIV patients. In a study by Janakiraman et al.,[8] a significant negative correlation between the CD4 count and albuminuria was found. In contrast, in an Indian study[12] no co-relation of renal histology with duration or severity of the disease was evident, as was also seen in our study.
Conclusions
Human immunodeficiency virus-related renal dysfunction is an important entity. In contrast to the previous studies where HIVAN was the predominant lesion, with prevalence ranging from 15.2 to 83%, the current study depicts that all varieties of glomerular and tubulointerstitial disorders can occur on histology. Hence, renal biopsy is indicated in renal dysfunction associated with HIV, for proper diagnosis and therapy.
Source of Support: Nil
Conflict of Interest: None declared.
References
- CDC. Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS among Adolescents and Adults. MMWR Recomm Rep. 1992;41(RR-17):1-19. 1993
- [Google Scholar]
- Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:669-73.
- [Google Scholar]
- U.S. Renal Data System, USRDS Annual Data Report. Bethesda, Maryland: National Institutes of Health, NIDDK; 1995.
- Acute renal failure in the course of HIV infection: A single-institution retrospective study of ninety-two patients and sixty renal biopsies. Nephrol Dial Transplant. 1999;14:1578-85.
- [Google Scholar]
- Renal failure in HIV/AIDS - clinical experience at Jodhpur, India - Int Conf AIDS. 2002:14. abstract no. B10333
- [Google Scholar]
- Renal disease in HIV seropositive patients in Nigeria: An assessment of prevalence, clinical features and risk factors. Nephrol Dial Transplant. 2008;23:741-6.
- [Google Scholar]
- Renal complications of human immunodeficiency virus type 1. Kidney Int. 1990;37:1571-84.
- [Google Scholar]
- Correlation of CD4 counts with renal disease in HIV positive patients. Saudi J Kidney Dis Transpl. 2008;19:603-7.
- [Google Scholar]
- Pathology of HIV-associated nephropathy: A detailed morphologic and comparative study. Kidney Int. 1989;35:1358-70.
- [Google Scholar]
- Renal lesions in AIDS: A biopsy and autopsy study. Indian J Pathol Microbiol. 1999;42:45-54.
- [Google Scholar]
- Spectrum of renal lesions in HIV patients. J Assoc Physicians India. 2000;48:1151-4.
- [Google Scholar]
- AIDS-related glomerulopathy: Occurrence in specific risk groups. Kidney Int. 1987;31:1167-73.
- [Google Scholar]







