Translate this page into:
Effect of short-term intravenous ascorbic acid on reducing ferritin in hemodialysis patients
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
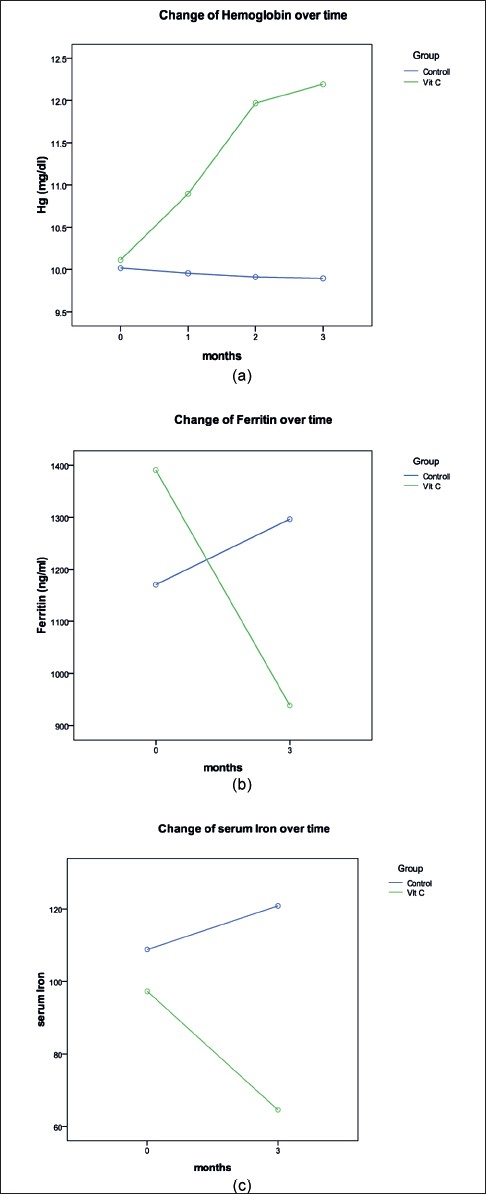
Resistance to recombinant erythropoietin (rEPO) in hemodialysis patients may be due to inadequate iron recruitment and defect in iron use. In this cross over randomized clinical trial, 30 hemodialysis patients with serum ferritin levels of ≥500 ng/ml, hemoglobin ≤11.0 g/dl, and transferrin saturation (TSAT) of 20% or less were administrated intravenous iron (50-100 mg/wk) and rEPO (120-360 U/kg/wk) for 6 months. Patients were excluded if there was a clear explanation for rEPO hyporesponsiveness. Patients were divided into two groups. Group1 received standard care and 500 mg of intravenous ascorbic acid (IVAA) with each dialysis session in the first week of each month for a total of 3 months. Group 2 received standard care only. After 2 month washout period, groups were crossed over. Each month hemoglobin (Hb) was assessed. Iron, TIBC (transferrin iron binding capacity), TSAT, iPTH (intact parathyroid hormone), liver enzymes, albumin and cholesterol levels were measured every 3 months. After 3 months of intervention, Hb significantly increased from 10.11 to 12.19 g/dl (P <0 0.001; 95% confidence interval [CI] 2.7-1.4) and TSAT increased from 18.9 to 28.1% (P = 0.008; 95% CI 0.09-3), while ferritin and serum iron declined significantly from 1391 to 938 ng/ml (P = 0.001; 95% CI 216-689), 97.2 to 64.6 (P = 0.001; 95% CI 14.8-50.4) in the study group. Change of Hb over time in IVAA group was significant (P < 0.0005). There were significant differences between two groups in change of Hb level over time (P < 0.0005) and treatment effect (P = 0.002). Baseline laboratory tests were similar in the two groups and there was no carry over effect at phase 2. We showed that low amount of IVAA could reduce ferritin level and enhance Hb and TSAT, suggesting improved iron utilization.
Keywords
Anemia
ascorbic acid
hemodialysis
Introduction
Anemia is common in patients with end-stage renal disease (ESRD) and is a risk factor for hospitalization and mortality.[1] The causes include blood loss caused by dialysis circuit, shortened red blood cell life, and poor production of erythropoietin, the most important reason for anemia.[2] Severe anemia is linked to adverse consequences[3] such as cardiac enlargement, ventricular hypertrophy, congestive heart failure, decreased cognitive and mental sharpness, and impaired immune responses.[4] Replacement therapy with recombinant erythropoietin (rEPO) is the key treatment for anemia. However, adequate storage of accessible iron in the body is required for inducing response to rEpo.[5]
The most common cause of EPO hyporesponsiveness in hemodialysis (HD) patients is absolute or functional iron deficiency. Other causes include chronic infection and inflammation, bone marrow malignancy, vitamin B12 and/or folate deficiency, secondary hyperparathyroidism, angiotensin-converting enzyme inhibitor therapy and aluminum toxicity.
In HD patients, ascorbic acid (AA) has an influence on improving sensitivity to EPO, either by increasing iron mobilization from tissue storage or by way of antioxidant effects.[6–10] In this study, we assessed whether short term treatment with intravenous AA (INAA) could diminish the high level of ferritin and raise Hb level.
Materials and Methods
This study was conducted in March 2010 at two HD centers (Vali-asr and Shahid Beheshti Hospitals) in the Iranian provincial capital of Zanjan. We investigated the effect of IVAA in 30 HD patients (56.6% men and 43.3% women, mean age of 52.2 years). The inclusion criteria were as follows: (1) on HD therapy for at least 6 months, (2) on rEPO for 6 months or longer at a dose of 120-360 U/kg/wk, (3) average Hb level of 11.0 g/dl or less for 3 months, (4) ferritin level greater than 500 ng/ml, (5) transferrin saturation (TSAT) of 20% or less, and (6) received maintenance intravenous iron (25-100 mg/wk). Exclusion criteria were (1) bone marrow malignancy, (2) myelodysplastic syndrome, (3) evidence of chronic infection, (4) hemochromatosis, (5) hemoglobinopathies, (6) evidence of significant bleeding (decrease in Hb level ≥2 g/dl during the past 3 months, and (7) sign of vitamin B12 and/or folate deficiency and intact parathyroid hormone (i-PTH) level >300 pg/ml.
Patients were also excluded if they developed bone marrow malignancy, myelodysplastic syndrome, hemochromatosis, or blood loss of 500 ml or greater during the 8-month study period. The study was approved by Institute Ethics Committee and patients gave signed consent. Study duration was 8 months (6 months to perform the study and 2 months wash-out period). Iron therapy was stopped for all the patients at the time of hyperferritenemia. During the study, dosage of EPO was held constant (between 120 and 360 U/kg/wk for each patient).
Blood samples for Hb, Hct (hematocrit), serum iron, TIBC, ferritin, and TSAT were obtained at baseline, monthly, and at the end of the study. In addition, iPTH, liver enzymes, albumin, and cholesterol were measured every 3 months.
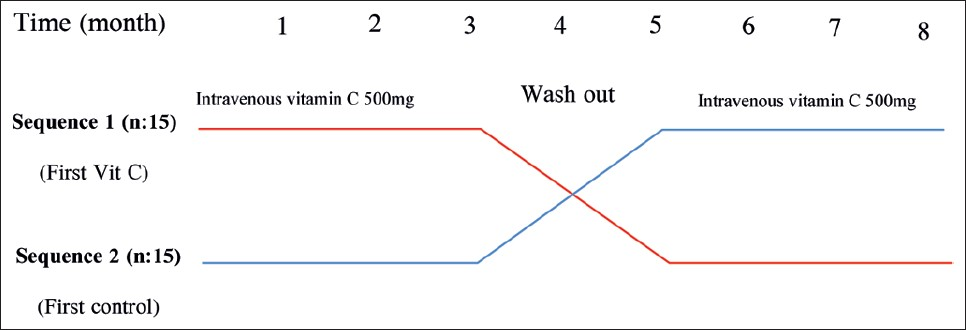
Patients were randomly divided into two groups. In the first phase of the study, group 1 (15 patients) received standard care and adjuvant therapy of 500 mg of IVAA after each dialysis session, three times a week, during the first week of each month (total of 1500 mg/month). Group 2 (15 patients) received standard care. After 3 months, we had 2 months of IVAA washout period. In the second phase of the study, groups were crossed over and IVAA was administrated to group 2 for 3 months similar to the first phase. Patients were assessed monthly for adverse events. A feedback form was used to assess the side effects of AA supplementation, such as dizziness, faintness, fatigue, flank pain, and headache. During the study, all patients were administered daily supplements of folic acid (5 mg) and vitamin B6, as well as 125 mg AA after each session of HD, and vitamin B12 (100 mg) per week. This trial was approved by committee of research ethics of Zanjan University of Medical Sciences. (Trial Registration Number: IRCT138904263325N3).
The analysis was on an intent-to-treat basis. All statistical analysis was performed using SPSS for windows (version 17) software. Means of quantitative variables were compared using Student's t-test between two groups. In the case of discontinuous variables, chi-square test was applied. Response to AA in the study group before and after intervention was assessed with paired samples t-test analysis. The repeated measures analysis of variance model was used to assess the effect of AA on change of Hb over time and difference of this effect between two groups (treatment effect and time × treatment interaction). All P-values were two-tailed and a P-value of <.05 was considered significant.
Results
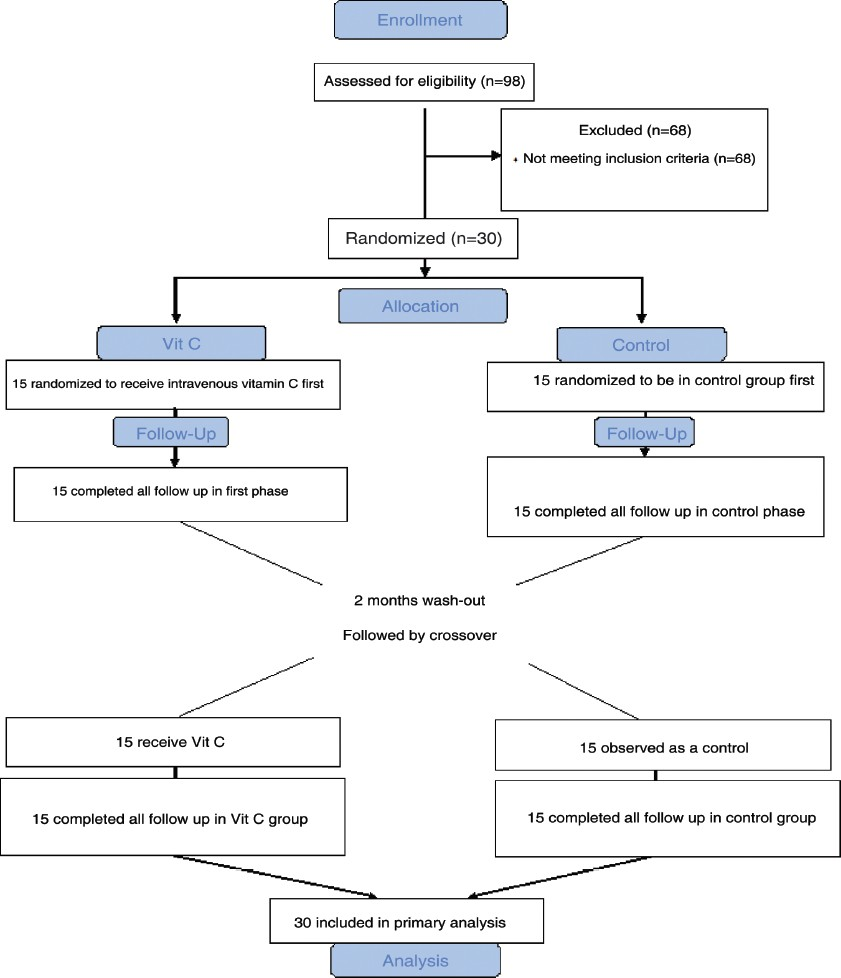
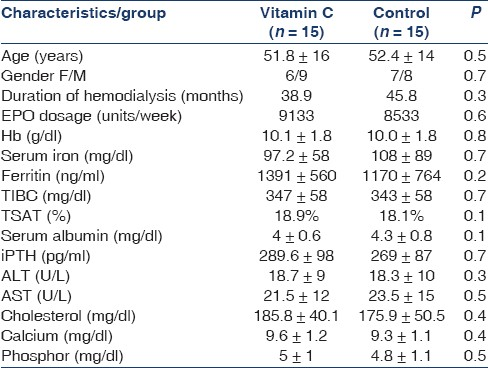
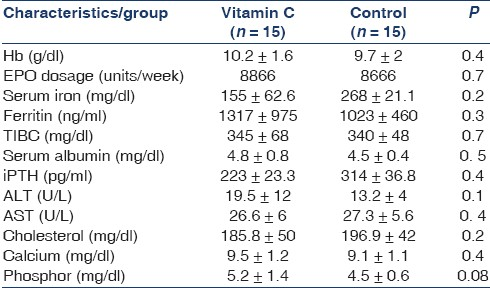
Ninety-eight HD patients were screened and 30 of them met inclusion criteria. These 30 patients (17 men and 13 women, with the mean age of 52.29 ± 16 years) were randomly chosen for two groups. All of them completed the study and were included in the analysis [Figures 1 and 2]. The mean dialysis duration was 44.5 months. All the patients were dialyzed for 4 hours three times a week, and the range of kt/v were comparable. Table 1 shows demographic and laboratory features of the participants in the two groups. In both groups from the beginning and during the two phases of study, the level of Hb, serum iron, serum ferritin, TIBC, TSAT, calcium, phosphor, aspartate aminotransferase and alanine aminotransferase, albumin, iPTH, cholesterol, and dosage of EPO were the same. Carry over effect was not seen at phase 2 of the study [Table 2]. After 3 months of the intervention, Hb increased from 10.11 to 12.19 g/dl (P < 0.001; 95% confidence interval [CI] 2.7-1.4), ferritin decreased from 1391 to 938 ng/ ml (P = 0.001; 95% CI 216-689), TSAT increased from 18.9% to 28.1% (P = 0.008; 95% CI 0.09-3),and serum iron decreased from 97.2 to 64.6 (P = 0.001; 95% CI 14.8-50.4) in the study group. All the above changes were significant. In the control group, change of Hb, ferritin, and other variables was not significant [Table 3]. Trend of Hb over time in intervention and control group is shown in Figure 3. Change of Hb over time in AA group was significant (F = 26.8, P < 0.0005), but in control group was not significant. There was significant difference between the two groups’ Hb level over time (F = 14.2, P < 0.0005). The results also showed a significant treatment effect (F = 10.9, P = 0.002) [Table 4].
- Flow chart of the study: Double-blind, two-period randomized cross-over study
- Study design
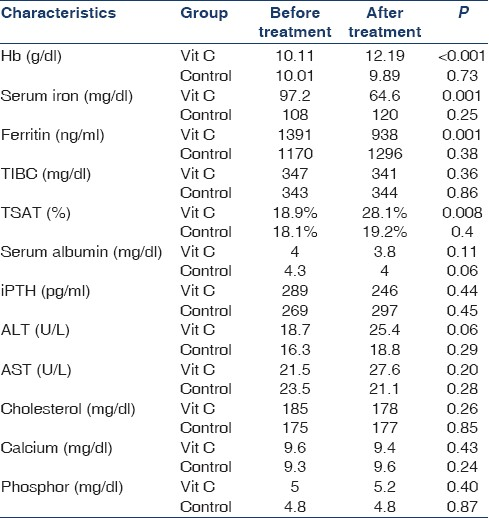
- Change of Hg (a), ferritin (b) and serum iron (c) in two groups over time (time effect). The effect of time is not the same for both groups and there is time and treatment interaction

Discussion
Management of anemia in patients with ESRD with EPO has been a major advance. The use of EPO has decreased the amount of blood transfusions and enhanced the quality of life in the ESRD patients.[11] EPO hyporesponsiveness is reported in HD patients.[1213] To resolve EPO hyporesponse, it has been recommended that the dosage of EPO be gradually increased.[6] However, the probable undesirable side effects related to the use of high erythropoietin doses[14] in theory has led physicians to reduce the dosage of erythropoietin.
Erythropoietin hyporesponsiveness has been described as the failure to achieve a Hb concentration target of 11 g/ dl, despite the use of an EPO dosage equal to at least 500 U/kg/wk.[15]
Iron deficiency is one of the reasons for anemia in patients with ESRD. Iron deficiency can be found more frequently with EPO administration.[16] However, HD patients may suffer from anemia, despite an iron overload. Administration of EPO can minimize iron overload.
In patients with kidney diseases, especially those getting dialysis, iron tends to be shifted out of circulation into storage tissues, making it less available for erythropoiesis. The syndrome of decreased accessibility of storage iron is referred to as “functional iron deficiency anemia.” This condition characterized by low TSAT, despite normal or increased total body iron storage (TSAT ≤ 20% and ferritin ≥500 ng/ml).[17] AA is involved in several phases of iron transport. It could release iron into the circulation and help induce EPO reaction.[1217–21]
In this study, we used IVAA in patients who had hyperferritenemia and EPO hypo responsiveness and found that the use of low amount of IVAA for short duration improved anemia and reduced the high level of ferritin. Attallah et al., in addition to standard care, administrated 300 mg of IVAA for 6 months with each session of dialysis for patients who were on EPO therapy for ≥6 months at a dose ≥450 U/kg/wk. In the AA group, Hb levels increased significantly and as a result the dosage of EPO was changed as well.[22] In this study, a total dosage of 4500 mg IVAA for 3 months was used compared with a total dosage 21,600 mg IVAA used for 6 months in Attalla's study. Our results showed significant increase in hemoglobin level from 10.11 to 12.19 g/dl.
Due to the loss of this water-soluble vitamin during the process of HD and inadequate intake from diet, HD patients are prone to subclinical AA deficiency. Therefore, routine AA supplementation for HD patients is suggested to be prescribed, whether or not they are receiving EPO.[23–27] According to the measurements used in the above study, our subjects did not have AA deficiency, because they were taking 125 mg of AA after each session of HD. So, one can assume that our patients’ storage of AA was not only low, but perhaps they even had sufficient supply. Therefore, they did not need to receive high amounts of IVAA supplement. In any case, we did not check the level of AA, so this could be considered as a limitation of our study.
Effect of AA on iron indices and ferritin concentration in three trials[82128] was different, while changes in ferritin level were moderate. Petrarulo and Giancaspro[29] and Ogi et al.[30] found a poor response even with higher and lower doses of IVVC, which were administrated for 3 months. There are other studies, too, that have not shown any benefit in the administration of AA.[31]
Erythropoietin dose have been adjusted by researchers in many trials, except in Giancaspro, et al.[32] study, in which the dosage was held constant. In our study, this measurement was also held constant.
Intravenous iron was given in all studies in either constant or adjusted doses except for the study by Sezer et al.[33] We did not administer iron during this study.
There are some concerns related to potential of secondary oxalosis with AA.[3435] It is particularly relevant to HD patients, who have increased serum oxalate levels.[36] We did not measure the plasma oxalate level, which is another limitation of our study.
The main differences of the presented study from the others are as follows: (1) the erythropoietin dosage was not changed to maximum and instead was kept constant; (2) at the time of hyperferritenemia, patients did not get IV iron; and (3) by considering the number of times and the amount that IVAA was administered, our patients used low dosage of AA (500 mg, three times a week, during the first week of each month, for a total of 3 consecutive months). The decision to use the lower dosage was based on limiting the probable collection of oxalate in patients, because we were not measuring oxalate levels.
We have found significant increases in Hb and Hct during the 3 months of IVAA treatment, while ferritin levels decreased. We did not discover any adverse events with this short-term dose of AA. However, further studies are needed to determine at what ferritin levels maximum response from AA treatment could be attained, and to ascertain the best dosage interval for optimal effect and minimal possible toxicity.
Acknowledgment
This work was supported by Iran's Zanjan University of Medical Sciences.
Source of Support: Iran's Zanjan University of Medical Sciences
Conflict of Interest: None declared.
References
- Revised European Best Practice Guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19(suppl 2):S1-47.
- [Google Scholar]
- Optimizing renal anemia management Benefits of early referral and treatment. Nephrol Dial Transplant. 2005;20(suppl 8):S22-6.
- [Google Scholar]
- The importance of early treatment of the anaemia of chronic kidney disease. Nephrol Dial Transplant. 2001;16(suppl 5):S45-9.
- [Google Scholar]
- Efficacy of oral iron therapy in patients receiving recombinant human erythropoietin. Am J Kidney Dis. 1995;25:433-9.
- [Google Scholar]
- 2004 Centers for Medicare and Medicaid Services: 2004 Annual Report, Clinical Performance Measures Project. Baltimore, MD, Department of Health and Human Services, Centers for Medicare and Medicaid Services, Center for Beneficiary Choices
- Effect of ascorbic acid administration in hemodialysis patients on in vitro oxidative stress parameters: Influence of serum ferritin levels. Am J Kidney Dis. 2003;42:158-66.
- [Google Scholar]
- Comparative study of intravenous ascorbic acid versus low-dose desferrioxamine in patients on hemodialysis with hyperferritinemia. J Nephrol. 2003;16:703-9.
- [Google Scholar]
- Randomized, crossover study of the effect of vitamin C on EPO response in hemodialysis patients. Am J Kidney Dis. 2003;41:1233-9.
- [Google Scholar]
- Intravenous iron preparations and ascorbic acid: Effects on chelatable and bioavailable iron. Kidney Int. 2005;67:1161-70.
- [Google Scholar]
- Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2000;58:1325-35.
- [Google Scholar]
- A parallel, comparative study of intravenous iron versus intravenous ascorbic acid for Erythropoietin-hyporesponsive anaemia in haemodialysis patients with iron overload. Nephrol Dial Transplant. 1998;13:2867-72.
- [Google Scholar]
- Results of the European Survey on Anaemia Management 2003 (ESAM 2003): Current status of anaemia management in dialysis patients, factors affecting epoetin dosage and changes in anaemia management over the last 5 years. Nephrol Dial Transplant. 2005;20(Suppl 3):S3-24.
- [Google Scholar]
- Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381-8.
- [Google Scholar]
- KDOQI; National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease. Am J Kidney Dis. 2006;47(suppl 3):S11-145.
- [Google Scholar]
- Parenteral ascorbic acid in hemodialysis patients. Curr Opin Clin Nutr Metab Care. 2008;11:741-6.
- [Google Scholar]
- Intravenous ascorbic acid as an adjuvant therapy for recombinant erythropoietin in hemodialysis patients with hyperferritinemia. Kidney Int. 1999;55:2477-86.
- [Google Scholar]
- Pharmacologic adjuvants to epoetin in the treatment of anemia in patients on hemodialysis. Hemodial Int. 2005;9:7-22.
- [Google Scholar]
- Oral use of iron with vitamin C in hemodialyzed patients. J Ren Nutr. 2003;13:47-51.
- [Google Scholar]
- Oral ascorbic acid as adjuvant to epoetin alfa in hemodialysis patients with hyperferritinemia. Am J Health Syst Pharm. 2004;61:2007-8.
- [Google Scholar]
- Efficacy and safety of oral versus intravenous ascorbic acid for anemia in hemodialysis patients. Nephrology (Carlton). 2005;10:336-40.
- [Google Scholar]
- Effect of intravenous ascorbic acid in hemodialysis patients with EPO-hyporesponsive anemia and hyperferritinemia. Am J Kidney Dis. 2006;47:644-54.
- [Google Scholar]
- Trace elements and vitamins in maintenance dialysis patients. Adv Ren Replace Ther. 2003;10:170-82.
- [Google Scholar]
- Effects of oral vitamin C supplementation on oxidative stress and inflammation status in haemodialysis patients. Nephrol Dial Transplant. 2005;20:1874-9.
- [Google Scholar]
- Is there a role for adjuvant therapy in patients being treated with epoetin? Nephrol Dial Transplant. 1999;14(suppl 2):50-60.
- [Google Scholar]
- Vitamin C status of patients with chronic renal failure, dialysis patients and patients after renal transplantation. Int J Vitam Nutr Res. 1997;67:262-6.
- [Google Scholar]
- Oxidative injury to erythrocytes, cell rigidity, and splenic hemolysis in hemodialyzed uremic patients. Ann Intern Med. 1975;82:460-5.
- [Google Scholar]
- Effect of intravenous ascorbic acid in hemodialysis and hyperferritinemia. Am J Kidney Dis. 2006;47:644-54.
- [Google Scholar]
- Intravenous ascorbic acid in hemodialysis patients with functional iron deficiency. Nephrol Dial Transplant. 2000;15:1717-8.
- [Google Scholar]
- Comparison of intravenous ascorbic acid versus intravenous iron for functional iron deficiency in hemodialysis patients [Article in Japanese] Nippon Jinzo Gakkai Shi. 2004;46:804-9.
- [Google Scholar]
- Effects of intravenous ascorbic acid on erythropoiesis and quality of life in unselected hemodialysis patients. J Nephrol. 2004;17:537-43.
- [Google Scholar]
- Intravenous ascorbic acid in hemodialysis patients with functional iron deficiency: A clinical trial. J Nephrol. 2000;13:444-9.
- [Google Scholar]
- Intravenous ascorbic acid administration for erythropoietin-hyporesponsive anemia in iron loaded hemodialysis patients. Artif Organs. 2002;26:366-70.
- [Google Scholar]
- Vitamin C intoxication and hyperoxalemia in chronic hemodialysis patients. Nephron. 1985;39:112-6.
- [Google Scholar]
- Oxalate metabolism in end-stage renal disease: The effect of ascorbic acid and pyridoxine. Nephrol Dial Transplant. 1988;3:28-32.
- [Google Scholar]
- Longterm, low-dose, intravenous vitamin C leads to plasma calcium oxalate supersaturation in hemodialysis patients. Am J Kidney Dis. 2005;45:540-9.
- [Google Scholar]







