Translate this page into:
The effect of niacin on serum phosphorus levels in dialysis patients
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hyperphosphatemia is common in patients with end-stage renal disease. Recent studies have shown that niacinamide and niacin achieve clinically significant reductions in serum phosphate in patients undergoing dialysis. The aim of the present study was to evaluate the serum phosphorus lowering effect of niacin in long-term hemodialysis patients. In this 8-week randomized, double-blind clinical trial, 37 patients were assigned to niacin or placebo with titration from 400 to 1000 mg daily. A 2-week washout preceded the switch from niacin to placebo or vice versa. The mean dose of niacin at the end of the 8-week treatment period was 750±200 mg/day. Serum phosphorus decreased from 6.66±1.40 to 5.96±0.87 mg/dL (P = 0.006) in the niacin-treated group after 8-weeks. However, the main reduction occurred at the beginning of study and seems not to be related to the phosphate-lowering effect of drug. In spite of a sharp increase in phosphorus level between w6 and w8 in patients on placebo, phosphorus values in drug-treated group showed nearly steady trend, presumably due to the inhibitory effect of niacin on phosphate absorption from gut. Niacin also increased the high density lipoprotein (HDL) cholesterol (P = 0.018). Our study suggests that niacin should be considered as adjunctive therapy for patients with hyperphosphatemia despite management with phosphate binders. The modest increase in HDL values may be another beneficial effect of this treatment.
Keywords
Hemodialysis
hyperphosphatemia
niacin
niacinamide
nicotinic acid
Introduction
Hyperphosphatemia has been associated with poor outcomes and mortality in chronic kidney disease (CKD)stage 5D, and high normal serum phosphorus levels have been associated with mortality in non-CKD patients and in CKD stage 3 patients.[1] Therefore, in patients with CKD stage 5D, Kidney Disease: Improving Global Outcomes (KDIGO) suggest lowering elevated phosphorus levels toward the normal range. The rationale for controlling serum phosphorus is based on epidemiological evidence suggesting that hyperphosphatemia is an important risk factor not only for secondary hyperparathyroidism, but also for cardiovascular disease.[2] Dietary phosphorus restriction and removal of phosphorus by dialysis are usually inadequate for controlling serum phosphate. Consequently, it becomes necessary to prescribe phosphate binders in order to reduce the amount of dietary phosphorus absorbed from the intestine. Currently available phosphate binders, while effective, are not considered optimal for treating hyperphosphatemia because they may lead to unacceptable adverse effects such as hypercalcemia (e.g., calcium carbonate) or potential toxicities (e.g., aluminum toxicity). Moreover, some binders are expensive (sevelamer and lanthanum carbonate). Thus, there is a need for phosphate lowering agents that overcome some of the defects of current therapy.
Niacin was first reported to decrease plasma cholesterol in 1955.[3] The major clinical use of niacin has been to increase high-density lipoprotein (HDL) cholesterol and reduce triglyceride levels. Animal studies have shown that niacinamide prevents an increase in serum phosphate in animals with renal failure by reducing sodium-phosphate 2b transporter expression in the jejunum and inhibiting intestinal phosphorus absorption.[45] As niacin is converted largely to niacinamide, it also inhibits intestinal phosphate absorption. Its major side effects are vasodilation and flushing, which appear to be mediated through prostaglandin production, and thus can be attenuated by premedication with aspirin.[6] However, recently the selective prostaglandin D2 receptor subtype 1 inhibitor laropiprant has been used in combination of niacin for reducing the flushing side effect of drug.[7]
Niacinamide, which is a circulating form of niacin, inhibits phosphate uptake by brush border membrane vesicles isolated from the rat small intestine.[45] Recent human clinical trials studies have also shown that niacinamide and niacin accomplish clinically significant reductions in serum phosphate in patients undergoing dialysis.[8–12] Niacin has potential advantages over current phosphate binders in that it does not need to be administered at the time of a meal.
The aim of the present study was to examine whether niacin lowers serum levels of phosphorus and intact parathyroid hormone (iPTH) in long-term hemodialysis patients.
Materials and Methods
This study was a randomized, double-blind clinical trial to evaluate the effectiveness of niacin versus placebo in reducing plasma phosphorus levels in HD patients. This study was approved by the Human Research Ethics Committee of the Arak University of Medical sciences (IRCT registration number: IRCT138812153492N1).
All 163 patients were screened for inclusion according to their most recent monthly plasma phosphorus value. Inclusion criteria were age >18 years, capable of giving informed consent, duration of HD >3 months, serum phosphorus levels 5-7 mg/dl, unchanged treatment protocol (calcium components and Vit D) during last 2 weeks and regular and steady dialysis program. Exclusion criterias were pregnancy, known liver disease, active peptic ulcer disease, treatment with carbamazepine, drug intolerance and necessity of changing treatment protocol because of safety criteria (such as severe hyperphosphatemia or two consecutive serum phosphorus levels > 7 mg/dl).
Forty-seven patients who met the inclusion criteria were enrolled. Patients were randomly assigned to placebo or niacin for 8 weeks. Niacin and placebo were packaged in identical 100-mg tablets, and dosages were titrated from 400 mg/d (taken as two tablets twice a day) to 1000 mg/d (five tablets twice a day) over 8 weeks. Every 2 weeks the dose was increased to 600, 800 and 1000 mg/d, respectively. After 8 weeks, patients underwent a 2-week washout period, followed by 8 weeks of the alternative therapy (from niacin to placebo, and vice versa). Patients who did not tolerate the dose increase remained on the highest tolerated dosage.
Aluminum-containing medications were withdrawn 2 weeks before starting the study. Concurrent binder therapy (calcium carbonate) and calcitriol were continued without any dosing adjustments during the study. Patients were counseled to take 100 mg aspirin 1 hr before niacin in case of flush symptoms. Compliance was confirmed by face-to-face interview.
All laboratory tests were performed at the Vali-asr Hospital laboratory. Blood samples were collected prior to a hemodialysis session. Calcium and phosphorus values were collected every 2 weeks; complete blood counts were collected every 4 weeks; total cholesterol, triglycerides, HDL cholesterol, low density lipoprotein (LDL) cholesterol and intact parathyroid hormone (iPTH) were collected at study start and end in each arms. The week 0 was used as a baseline for comparison to the final study (week 8) value for all lab parameters. PTH was measured using the intact-PTH electrochemiluminescence immunoassay.
Glucose and liver enzymes values were measured every 4 weeks to look for adverse drug effects.
Statistical analysis was performed using SPSS 17.0. The primary end point was the change in serum phosphorus from the first to the last week of each arm. Predefined secondary end points were the change in iPTH, platelets and lipid profile (HDL, LDL, triglycerides). P<0.05 indicated statistical significance.
Results
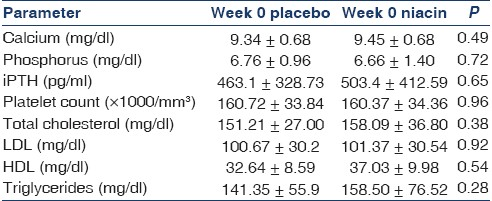
Forty-seven patients were consented and randomized as placebo or niacin to participate in the study. Five in the placebo arm were withdrawn prematurely because of the need for change and adjustment of phosphate binder treatment protocols (prescription of aluminum hydroxide) and five patients in the niacin arm were withdrawn due to intractable flushing and gastrointestinal upset. All withdrawals occurred in the beginning of study and before the crossover. Thirty-seven patients (18 men; age, 57±11 years) remained in study until the end. Patients baseline characteristics are shown in Table 1.
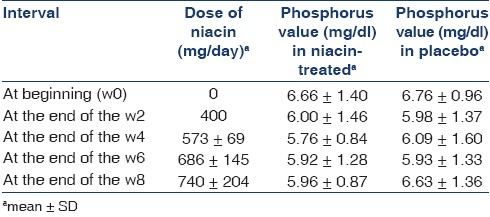
The mean dose of niacin at the end of the 8-week treatment period was 740±204 mg/day. The minimum and maximum doses of niacin were 400 and 1000 mg/day respectively. The mean value of phosphorus and the mean dose of niacin at the end of the 2, 4, 6 and 8-week treatment period in both drug-treated and placebo groups have been shown in Table 2.
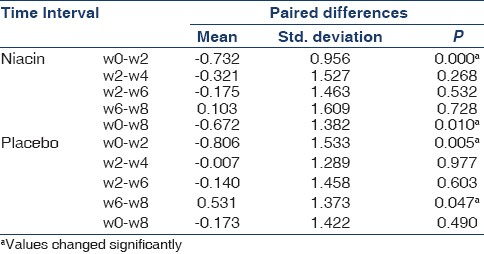
There were statistically significant decrements in serum phosphorus levels throughout the first 2 weeks of study period in both niacin-treated and the placebo groupsth . After week 2 no significant changes occurred in phosphorus levels in every 2 weeks serial measured values in the niacin-treated group [Table 3]. However, there was a significant increase in phosphorus value between w6 and w8 in the placebo arm, despite being relatively stable in the two previous measuring (P=0.003). This acute increase in phosphorus levels led to significant differences in the mean values of phosphorus at 8 weeks between drug-treated and placebo groups (P=0.039). Changes in serum phosphate concentrations are illustrated in Figure 1. Also, as has been shown in the Table 2, serum phosphorus decreased from (6.66±1.40) to (5.96±0.87) mg/dL (P=0.006) in the niacin-treated group at the 8-week treatment.
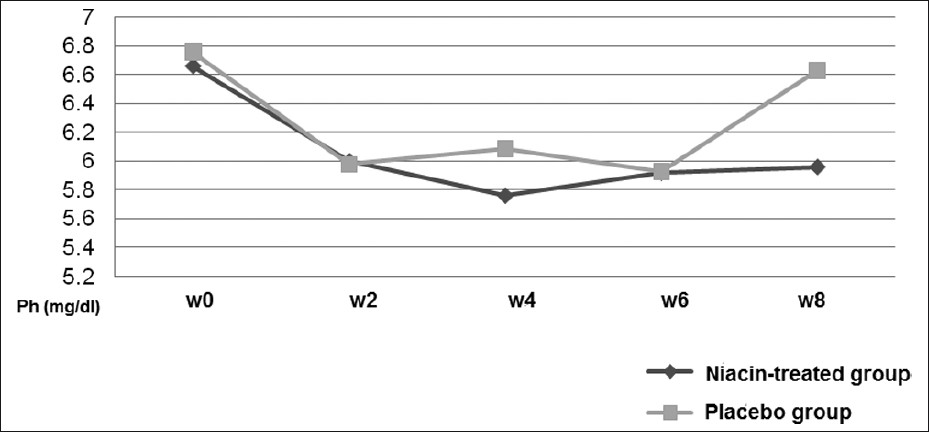
- Changes in serum phosphate concentrations
The mean values of phosphorus of week 8 have been compared in two groups of niacin-treated and placebo [Table 4]. As has been shown, there was significant difference between phosphorus levels in w8 for patients able to be titrated to more than 800 mg/day niacin (57%) and placebo; however, no meaningful difference was observed in the lower doses (P=0.006).

Additionally, no statistically significant changes were demonstrated for serum calcium or iPTH values [Table 5].
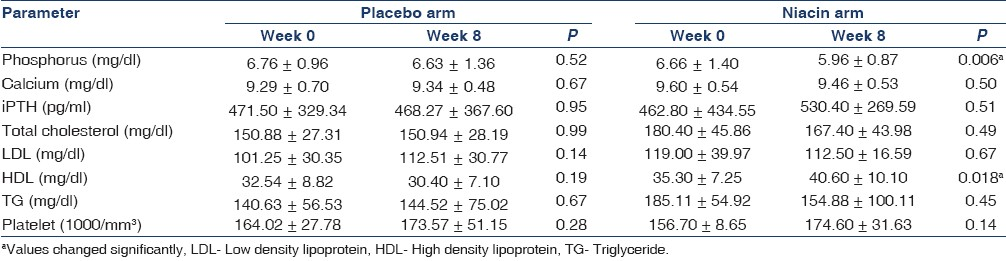
Niacin increased HDL cholesterol from 35.3±7.26 to 40.6±10.1 mg/dl (P=0.018) in the high-dose group; however, no significant changes occurred in the low-dose or placebo groups (P>0.05). There were no statistically significant changes in serum total cholesterol, LDL cholesterol and serum triglyceride levels throughout the study period [Table 5].
There was no statistically significant changes in mean platelets levels among the niacin-treated participants (P=0.240). The most common adverse events which, possibly related to niacin treatment were flushing and gastrointestinal symptoms. Severe flushing which led to discontinuing of the drug, were observed in three patients. However, 40% (15/37) of cases suffered from mild to moderate flushing, which were major barrier for drug titration. As pointed before, gastrointestinal symptoms were the reason for the withdrawal of two patients from the study. Mild pruritus was encountered in seven patients. All severe symptoms related to niacin were observed at the commencement of treatment.
Discussion
In spite of introduction of new phosphate-bindering drugs, improved membrane technology and quality improvements of dialysis, control of hyperphosphatemia remains a major challenge in hemodialysis patients’ care. In recent years, introduction of niacin and niacinamide derivatives as phosphate-binder agents has given birth to new hopes to solve the problem of hyperphosphatemia in advanced renal failure. Some studies have evaluated niacinamide for demonstrating the phosphate-lowering effect of these derivatives.
The first clinical trial was done by Takahashi et al.[8] They started niacinamide at 500 mg/d in 65 hemodialysis patients, which was titrated to a maximum dose of 1750 (1080±370) mg/day. During the 12-week treatment, phosphorus levels fell from 6.9±1.5 to 5.4±1.3 mg/dL (P<0.001) with no meaningful change in serum calcium levels, while intact parathormone decreased significantly.
Muller et al.,[9] used extended-release niacin (Niaspan) with a mean dose of 1470±110 mg/day in 20 dialysis individuals for the 12-week. The goal was to tolerate at least 1000 (1470±110) mg/d of Niaspan, for which 17 individuals qualified. Serum phosphorus decreased from 7.2±0.5 to 5.9±0.6 mg/dl (P<0.015) and HDL cholesterol increased from 40±3.2 to 59±5.5 mg/dL (P=0.0005). They demonstrated no significant change in serum calcium or iPTH levels.
Cheng et al.,[10] used niacinamide in a randomized, double-blind, placebo-controlled crossover trial with 33 individuals on hemodialysis. Niacinamide dosages were titrated from 500 mg/day to 1500 mg/day. Serum phosphorus decreased from 6.26±1.28 to 5.47±1.49 mg/dL (P=0.02) with niacinamide; while there was no significant change in the placebo group. HDL cholesterol increased from 50±17 to 61±21 mg/dL with niacinamide but not placebo. In this study there were no statistically significant changes to serum calcium or iPTH levels.
Maccubbin et al.,[13] performed a post hoc data analysis of serum phosphorus concentrations among 1547 patients who had dyslipidemia and were randomly assigned to treatment with extended release niacin (ERN; 1 g/d for 4 weeks and dose advanced to 2 g/d for 20 weeks) combined with the selective prostaglandin D2 receptor subtype 1 inhibitor laropiprant (ERN-L) (n=761), ERN alone (n=518), or placebo (n=268). They demonstrated that ERN-L and ERN alone caused a sustained approximately 11% reduction in serum phosphorus [–0.41 mg/dl (–0.46 to –0.37 mg/dl)], accompanied by lowering of the calcium-phosphorus product, without raising serum calcium concentrations, which is unaffected by estimated GFR ranging from 30 to 90 ml/min per 1.73 m2 (i.e., stages 1 through 3 chronic kidney disease).
Our study used short-acting oral 100 mg tablet. We recorded a sharp decrease in phosphate values in the first 2 weeks of the study in both groups, which have been justified as the initial reduction of phosphorus levels in control group was intriguing, possible effect of study participation. Tracing phosphate values in consecutive weeks showed changes in phosphate levels at 2, 4, 6 and 8 weeks in the treatment group. However, significant increase was observed in phosphate values at w8 in patients on placebo. Our impression was that this gap is due to inhibitory effect of niacin on rising serum phosphorus after meal consumption, because drug-treated patients were on average dose of 740±204 mg/day of niacin at the end of week 8. Additionally, by comparing the mean value of phosphate at week 8 and niacin dosage, these results were reproducible only in cases able to be titrated to more than 800 mg/day of niacin (n=21, 57%). On the other hand, it seemed doses lower than 800 mg/day are not effective in short term treatment. Our results were different from Sampathkumar et al.,[11] where they have shown significant phosphorus reduction on a 375 mg/day extended-release niacin in 30 out of 34 individuals. In all other studies, the minimum prescribed dosage was 1000 mg/day of niacin or niacinamide formula. Restrepo Valencia et al.,[12] observed that niacin generated a significant decrease in serum phosphate levels at 8 months, but not at 4 months. They assumed that the required dose the patients should take inorder to achieve the desired effect may be 1,000 mg, since at that time 100% of them were already receiving that dose.
Also, we detected significant increase in HDL cholesterol level, which is consistent with previous reports.[8–13] In our study LDL levels decreased, but the changes were not statistically significant. Also, there were no statistically significant changes to serum calcium. In our study, intact PTH levels were not significantly changed despite decrease in phosphate levels. However, a longer follow-up period and a higher number of patients are required to really obtain data with higher statistical power. In this respect our data was consistent with results of Muller[9] and Cheng.[10] Takahashi,[8] however, reported significant decreases in serum iPTH and alkaline phosphatase levels. We did not detect any significant changes in platelets counts. Our data was consistent with finding from most of the previous studies.
On a contrasting note, all previous investigations were implemented by extended-release form of the drug.[911–13] Niacin marketing in our country is limited to short-acting form of oral 25 mg and 100 mg tablets and therefore one of the main barriers for satisfactory compliance was a number of pills patients had to take. Also, one of the other limitations in our study was the assessment of compliance by face-to-face interviews and relience upon patients’ remarks.
The main troublesome constraint in our study was the erratic changes in phosphorus values caused by food variety. Although, we had a dietician on hand to counsel patients almost throughout the study period, the influence of food variety effect is irrefutable especially because of the short duration of the study.
Conclusions
Our study suggests niacin may emerge as a safe, low-cost therapy in combination with other phosphate binders for phosphate control. The modest increase in HDL values may be another beneficial effect of this treatment. However, larger and longer term controlled trials are needed to establish the optimal dosage and the clinical significance of niacin treatment.
Source of Support: Department of Internal Medicine, Nephrology-Internal Medicine, Endocrinology, Arak Medical Science University, Arak, Iran.
Conflict of Interest: None declared.
References
- KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1-130.
- [Google Scholar]
- Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208-18.
- [Google Scholar]
- Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955;54:558-9.
- [Google Scholar]
- Nicotinamide inhibits sodium dependent phosphate cotransport activity in rat small intestine. Nephrol Dial Transplant. 1999;14:1195-201.
- [Google Scholar]
- Nicotinamide prevents the development of hyperphosphatemia by suppressing intestinal sodium-dependent phosphate transporter in rats with adenin-induced renal failure. Nephrol Dial Transplant. 2005;20:1378-84.
- [Google Scholar]
- Release of markedly increased quanitites of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins. 1989;38:263-74.
- [Google Scholar]
- Review of extended-release niacin/laropiprant fixed combination in the treatment of mixed dyslipidemia and primary hypercholesterolemia. Vasc Health Risk Manag. 2009;5:901-8.
- [Google Scholar]
- Nicotinamide suppresses hyperphosphatemia in hemodialysis patients. Kidney Int. 2004;65:1099-104.
- [Google Scholar]
- Niacin lowers serum phosphate and increases HDL cholesterol in dialysis patients. Clin J Am Soc Nephrol. 2007;2:1249-54.
- [Google Scholar]
- A randomized, double-blind, placebo-controlled trial of niacinamide for reduction of phosphorus in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1131-8.
- [Google Scholar]
- Extended release nicotinic acid-a novel oral agent for phosphate control. Int Urol Nephrol. 2006;38:171-4.
- [Google Scholar]
- Safety and effectiveness of nicotinic acid in the management of patients with chronic renal disease and hyperlipidemia associated to hyperphosphatemia. Nefrologia. 2008;28:61-6.
- [Google Scholar]
- Hypophosphatemic effect of niacin in patients without renal failure: A randomized trial. Clin J Am Soc Nephrol. 2010;5:582-9.
- [Google Scholar]







