Translate this page into:
Spectrum of acute kidney injury in the Himalayan region
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Acute kidney injury (AKI) is common in hospitalized patients and is an important cause of mortality. This is a descriptive study of AKI in patients from Himachal Pradesh, India, located in Western Himalayan region. Over a period of 1 year, 102 patients with clinical and laboratory evidence of azotemia were included. Out of 102 patients, 84.3% had community acquired AKI and 15.7% had hospital acquired AKI. Medical causes were leading contributors (85.3%), with septicemia being the main factor (33.3%). Multiorgan failure was present in 59.8% patients. The overall mortality was 29.2%, and community acquired AKI was associated with higher mortality as compared to hospital-acquired AKI (22.5% vs 6.7%). AKI is still common in community and associated with high mortality. Septicemia, volume depletion and nephrotoxins were the leading cause of AKI in our study. Our study highlights the presence of hypotension, multiorgan failure and oliguria with mortality. Community-acquired AKI had higher mortality than hospital-acquired AKI.
Keywords
Acute kidney injury
community-acquired acute kidney injury
hospital-acquired acute kidney injury
Western Himalayas
Introduction
Acute kidney injury (AKI) is characterized by rapid (over hours to days) decline in glomerular filtration rate, retention of nitrogenous waste products and perturbation of the extracellular fluid volume, electrolytes and acid-base homeostasis.[1] AKI constitutes approximately 5% of hospital admissions and up to 30% of admissions to intensive care units.[1] Although reliable statistics on the prevalence of AKI are not available, statistics on referrals to dialysis units suggest that the condition is more common in India as compared to the West.[2] AKI can result from decreased renal perfusion without cellular injury, an ischemic, toxic or obstructive insult to the renal tubule, a tubulointerstitial process with inflammation and edema or primary reduction in the filtering capacity of the glomerulus.[2] The mortality rate among patients with AKI approaches 50% and has changed little over the past 15 years.[1] This study was conducted in a tertiary care hospital providing nephrology services in Himachal Pradesh, India, located in Western Himalayas to assess clinico-etiological aspects, risk factors, clinical course and outcome of AKI. This study is first of its kind from this region.
Materials and Methods
This was a prospective study, done over a period of 1 year in a tertiary care hospital. All adult (>18 years) patients admitted to this hospital were included. All one hundred and two patients with clinical (uremic symptoms or oliguria or anuria of recent onset) and laboratory evidence of azotemia (urea and creatinine above 40 mg/dl and 1.5 mg/dl, respectively) were eligible. Adult patients who developed kidney injury after at least 24 hours of admission were referred as hospital-acquired AKI. Patients who had AKI on admission were considered as community-acquired AKI. Patients with chronic renal failure, acute on chronic renal failure and those not willing to participate were excluded from this study. The patients included in the study were explained in detail about the purpose of the study and an informed consent was taken. Evaluation included a detailed history, physical examination and laboratory investigations. Patients were followed up until discharge or death. Each patient was looked for the complications of AKI-like fluid overload, hypertension, electrolyte abnormalities, metabolic acidosis, uremic complications, bleeding, neurological abnormalities and infections. Hemodialysis was instituted as and when required. Kidney injury was listed as a cause of death if patient exhibited evidence of severe uremia, hyperkalemia or volume overload secondary to oliguria. Patients were classified as oliguric (urine output<500 ml/day) and non oliguric (urine output>500 ml/day) during the azotemic phase.The study has been approved by institutional ethics committee. Chi-square test was used for statistical analysis.
Results
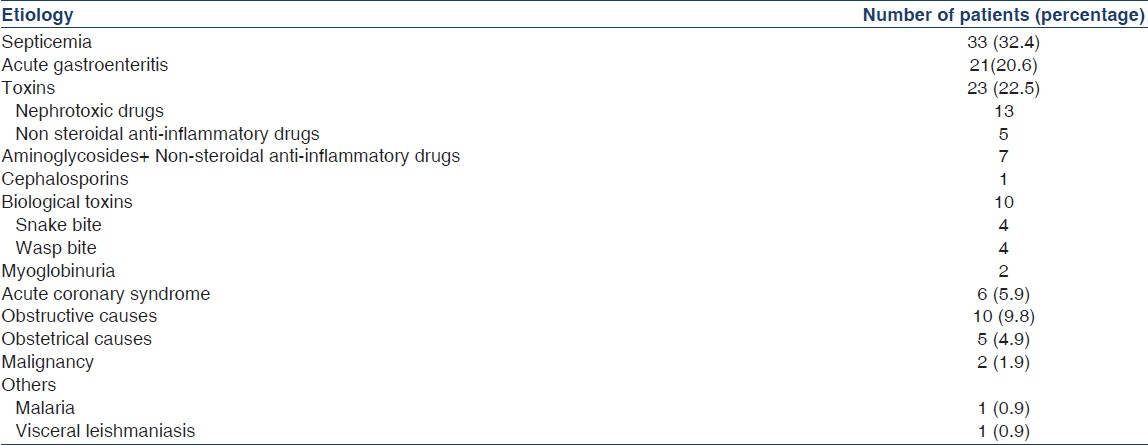
One hundred and two patients who satisfied the inclusion criteria were included. A total of 19,248 admissions were recorded in the hospital during the year of study. The prevalence of AKI was 0.53%. Community-acquired AKI was observed in 86 patients (84.3%) and hospital-acquired kidney injury in 16(15.7%) patients. The mean age of these patients was 48.96±18.3 (range 18 to 85) years. The number of males was 63(61.8%) and 39 were females (38.2%). The mean duration of hospital stay was 9.41±7.3 days with a range from 1 to 34 days. Out of the 102 patients, 87 were admitted with medical causes (85.3%), 10 with surgical causes (9.8%) and 5 with obstetrical causes (4.9%). Table 1 shows the causes of AKI. The coexisting conditions observed in AKI were observed in 87(85.3%) patients and is shown in Table 2. Multiorgan failure was present in 61 (59.8%) patients and single organ failure in 41(40.2%) patients. Twenty-eight (45.9%) patients with multiorgan failure died. All the patients who died had two or more etiological factors for AKI. No mortality was observed in patients having single organ failure. Active urinary sediment was noted in 54 patients and mortality rate in them was 56%. Kidney biopsy was done in nine patients which revealed acute tubular necrosis (ATN) in 4: snake bite 2, wasp bite 1, drugs 1 (NSAIDs+aminoglycoside 1); allergic interstitial nephritis (AIN) in 1 (cephalosporins); ATN+AIN in 1 (wasp bite); diffuse proliferative glomerulonephritis (DPGN) in 2 and crescentic glomerulonephritis in 1. In the remaining majority of patients either the consent for kidney biopsy was not available or the patients had improved. Forty-two patients did not have active sediment in urine. The mortality in them was 44% (P>0.05). During hospital stay 64 patients (66.7%) developed documented or suspected infection. The rate of infection was 62% in those who survived and 78% in those who died (P>0.05). Bacterial cultures from blood and urine were positive in 28 patients. Blood culture alone was positive in 10 patients. The mortality rate with positive blood culture was 48% as compared to that of 39% where blood culture was negative (P>0.05). In the study, 64 patients required vasopressor support and 26 (40.6%) died whereas 2 (5.3%) out of 38 patients died who did not receive vasopressor support (P<0.05). Fifty-four patients (53%) received dialysis, were treated with peritoneal dialysis (PD) and 50 with hemodialysis (HD). On an average each patient treated with HD received 2.5±1.5 (range 1-7) sessions of HD. 25 (46.3%) of the patients who received dialysis died. In this study, 16 patients had nonoliguric AKI and mortality in nonoliguric AKI was 11% as compared to 46% in the oliguric patients (P<0.05). Severe metabolic acidosis was present in 12 patients (11.8%). All of them died despite dialysis. Hyperkalemia was noted in 10 patients (9.8%) who also had metabolic acidosis. All of them died despite dialysis support. Pre-renal AKI was present in 21.6 (20.6%), intrinsic renal in 71(69.6%) and post-renal in 10(9.8%). Out of 102 patients 28 died (29.2%) and 68 patients recovered renal function (70.8%). In 6 patients (5.9%) outcome could not be ascertained as they were either discharged on request or left against medical advice before recovery. The mortality was 6.7% in hospital acquired AKI and 22.5% in community acquired AKI (P<0.05).

Discussion
The present study was conducted in a tertiary care hospital a hilly state in western Himalayas. Out of a total 102 patients with AKI, 84.3% had community acquired AKI and 15.7% patients had hospital acquired AKI. Higher incidence of community acquired AKI in our study is multifactorial. It is due to endemic malnutrition, poor socioeconomic conditions, hot climate (resulting in peripheral vasodilatation), which in conjunction with excessive sweating predisposes to hypovolumic insults. The study revealed that our patients are more than a decade younger than in the west but comparable with most of Indian studies. Medical causes contributed to AKI in 85.3%, surgical causes in 9.8% and obstetric causes in 4.9% of patients. A study from south India showed 87.6% AKI in medical patients, 8.9% and 3.4% AKI in surgical and obstetrical patients respectively[3] and similar observations have been made previously.[4] The pattern is however changing, surgical causes are increasing due to more trauma and major surgeries and obstetrical causes are decreasing because of better care in obstetrics.[56] The low percentage of AKI in surgical patients in our study could be attributed to non performance of open heart and pancreatic surgery in our institution during the study period.
Among the causes leading to AKI, intrinsic renal causes were seen in 69.6%, pre-renal in 20.6% and postrenal causes were present in 9.8% patients. Septicemia was observed in 33.3%, acute gastroenteritis(leading to volume depletion) in 20.6%, toxic causes (nephrotoxic drugs+biological toxins) in 25.5%, obstructive uropathy in 9.8%, toxemia of pregnancy in 3.9%, cardiogenic shock in 5.9%, cerebral malaria and visceral leishmaniasis in one patient each. Eleven out of 34 patients who died were aged 60 years and above accounting for 32.4%. The literature regarding the development of AKI in elderly with septicemia is variable.[7–9]
In the present study drugs were responsible for the development of AKI in 16 patients (15.7%). In all the patients it was hospital acquired AKI. Drug-related causation was implicated in 18.3% of AKI cases reported in a one year survey. In India a study reported a 20% incidence of drug induced AKI with a larger proportion (40%) being secondary to aminoglycoside usage.[10] NSAID'S, ACEI'S, radiocontrast media and aminoglycosides are labeled as “internists nephrotoxic quartet” as these are common nephrotoxic drugs. The kidney is an organ characterized by a large blood supply which ensures a high level of toxicant delivery over a period of time. The extensive resorptive capacity of the tubule with specialized transporters promotes cellular uptake of the toxicant. Concentrating capability of the tubule produces the high concentrations in the medullary lumen and interstitium.
In the present study, 9.8% patients had obstructive uropathy. Whereas trauma, drugs and cardiovascular surgery are the leading causes of surgical AKI in the developed countries, obstructive uropathy constitutes a major cause of surgical AKI in certain tropical areas. The obstetrical causes of AKI were 5.2% in this study which were comparable with the figure of 6% by an Indian study.[4] Improvements in obstetrical care have led to a decline in the incidence of obstetric AKI from 22% of all AKI in 1960s to 8% in 1990s.[11]
Mean duration of hospital stay was 9.4 days. An overall mortality was 29.2% in this study. Community acquired AKI was associated with increased mortality as compared to hospital-acquired AKI (22.5% vs 6.7%). Mortality was high in patients having AKI due to medical causes as compared to patients with AKI resulting from surgical and obstetrical causes. Possible explanation for the lower mortality in surgical and obstetrical patients is that they did not have multiorganfailure which was common among patients with medical AKI. There was no significant difference in mortality in terms of age and gender. Presence of cardiovascular diseases, neurological diseases at the time of presentation to the hospital were associated with higher mortality. Respiratory diseases, malignancy, hypertension, diabetes were not associated with increased mortality. The mortality was high in patients with multiorgan failure as in other studies.[61213] The mortality was highest in the renal category of AKI. Presence of hypotension was associated with significant mortality. Hypotension has been observed to be an adverse prognosticator in AKI.[10] Mortality was high in patients who required vasopressor support and amongst those who required dialysis therapy. Vasopressor support as well as dialysis support are considered a prognostic factor for outcome in AKI patients.[14] Severe metabolic acidosis and hyperkalemia were associated with adverse outcome. Patients with oliguria and active urinary sediments had poor outcome. Urine output is an established prognosticator in AKI.[15–17] Occurrences of infections after the development of AKI were associated with a poor outcome. Infections are more likely in these patients because of an impaired immune system.
Conclusions
In conclusion, AKI is still common in the community and is associated with high mortality. Septicemia, volume depletion and nephrotoxins were the leading cause of AKI in our study. Presence of hypotension, multiorgan failure, oliguria and active urine sediment and complications in the form of severe metabolic acidosis and hyperkalemia and patients with renal category of AKI were associated with increased mortality.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Acute renal failure. In: Braunwald E, Kaspar DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison's Principles of Internal Medicine (17th ed). New York: McGraw-Hill; 2008. p. :1752-61.
- [Google Scholar]
- Epidemiologic trend changes in acute renal failure-a tertiary center experience from South India. Ren Fail. 2006;28:405-10.
- [Google Scholar]
- A clinical profile of “Acute Renal Failure”. J Assoc Physicians India. 1998;46:130.
- [Google Scholar]
- Risk factors and outcome of hospital-acquired acute renal failure Clinical epidemiologic study. Indian J Nephrol. 1987;83:65-71.
- [Google Scholar]
- A profile of Acute Renal Failure patients in Western Rajasthan. J Assoc Physicians India. 1999;47:102.
- [Google Scholar]
- Treatment-related acute renal failure in the elderly: A hospital-based prospective study. Nephrol Dial Transplant. 2000;15:212-7.
- [Google Scholar]
- Drug-induced nephrotoxicity: the crucial role of risk factors. Postgrad Med. 1996;100:83-4. 87-8, 91 passim
- [Google Scholar]
- Spectrum of acute renal failure in North India. J Assoc Physicians India. 1978;26:147-54.
- [Google Scholar]
- Short and long term outcome in a consecutive series of 419 patients with acute dialysis-requiring renal failure. Scand J UrolNephrol. 1993;27:453-62.
- [Google Scholar]
- Acute renal failurein intensive care units– causes, outcome and prognostic factors of hospital mortality; a prospective multicentre study.French Study Group on Acute Renal Failure. Crit Care Med. 1996;24:192-9.
- [Google Scholar]
- Easy and early prognosis in acute tubular necrosis: a forward analysis of 228 cases. Nephron. 1989;51:307-13.
- [Google Scholar]
- Hospital-acquired renal insufficiency: A prospective study. Am J Med. 1983;74:243-8.
- [Google Scholar]
- A clinical index to predict survival in acute renal failure patients requiring dialysis. Am J Kidney Dis. 1988;24:254-9.
- [Google Scholar]







