Translate this page into:
Extended spectrum beta lactamase peritonitis: Time for innovation?
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Extended spectrum beta lactamase (ESBL) producing bacteria that are capable of hydrolyzing even third generation cephalosporin are emerging as a potent threat. We report a seven-year-old child on continuous ambulatory peritoneal dialysis, who developed ESBL producing Klebsiella pneumoniae peritonitis. The bacterium was resistant to the usual intraperitoneal antibiotics. We successfully treated the child with intravenous meropenem along with oral cotrimoxazole. The case highlights the menace of ESBL peritonitis, as also a need for the development of guidelines for such a scenario, which is becoming increasingly common in India.
Keywords
Continuous ambulatory peritoneal dialysis
extended spectrum beta lactamase
pediatric
peritonitis
Introduction
Peritonitis remains an important complication of peritoneal dialysis (PD), despite improvement in connectology.[1] The problem has been further compounded by the growing menace of antibiotic resistance, which is a global problem, and is especially important for children on PD because of the combination of susceptible patients and high antibiotic selection pressure. The emergence of extended spectrum beta lactamase (ESBL)-producing gram-negative Enterobacteriaceae (primarily Escherecia coli, Klebsiella pneumoniae, and Proteus Mirabilis) is challenging as it is efficient and inactivates even the third generation of cephalosporin. These Enterobacteriaceae frequently present a therapeutic dilemma, as they are often resistant to the intra-peritoneal antibiotics recommended by the International Society of Peritoneal Dialysis (ISPD).[1] We hereby present a case of ESBL producing K. pneumoniae (KP) peritonitis in a child on continuous ambulatory peritoneal dialysis (CAPD) which to the best of our knowledge is the first such reported case in the pediatric age group, and thereafter, briefly discuss the therapeutic dilemma of treating such cases.
Case Report
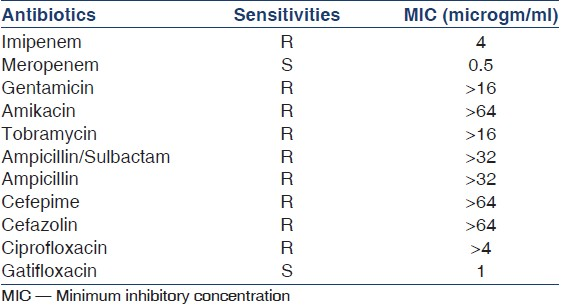
A-seven-year-old boy on CAPD presented with fever, vomiting, and pain in the abdomen. Peritonitis was suspected based on the clinical signs as well as the cloudy PD fluid effluent. This was confirmed by PD fluid cytology, which showed a cell count of 1020/ml with 92% neutrophils. He was started on intraperitoneal (IP) ceftazidime and vancomycin, but failed to show any signs of clinical improvement. At 72 h, the PD fluid culture was confirmed to be Klebsiella pneumoniae (KP)-secreting ESBL (confirmed by the double disk synergy test). Culture sensitivity showed it to be resistant to not only cephalosporins, but also to imipenem, ciprofloxacin, and aminoglycosides. It was sensitive only to meropenem, polymixin, gatifloxacin, and trimethoprim [Table 1]. As none of the sensitive antibiotics were featured in the ISPD recommended list of IP antibiotics, he was started on intravenous meropenem along with oral co-trimoxazole. The child showed excellent response, becoming asymptomatic in 72 h. The antibiotic was continued for 2 weeks and a repeat PD fluid analysis showed a normal cell count and sterile culture. At the 12 month follow-up, the child continues to be successfully managed on CAPD, with no significant change in his membrane character as per his repeat Peritoneal Equilibration test (PET) results.
Discussion
Plasmid-mediated ESBL was first described in 1983 among Serratia marcescens and KP.[2] As a result of point mutations in the genes coding the plasmid-mediated enzyme, they are capable of hydrolyzing all beta lactamase, except carbapenams. Among gram-negative bacteria this has become the most important mechanism of resistance to beta lactam agents, and worldwide there has been a significant rise in its incidence.[3] Even in the tertiary care hospital in Kolkata, where the index case was treated, the incidence of ESBL among Enterobacteriaceae has increased from 35% in 2006 to 52% in 2010. Although initially ESBLs were more confined to hospital-acquired infection, the recent trend has shown a worrisome increase in its prevalence among community-acquired infection.[45] Despite this, there are very few published reports on ESBL peritonitis among patients on PD, with none from the pediatric age group.[67] To some extent, this can probably be attributed to difficulties associated with identification of ESBLs.[8] Controversy exists regarding choice of the optimal laboratory method for detecting ESBLs. Any method that relies exclusively on phenotypic expression of beta lactamase fails to detect all cases of ESBLs. The current recommendation advocates the use of the double disk synergy test, and its routine use should decrease the chance of missing an ESBL. In this, the diameters of the inhibition zones are compared between the cefotaxime/ceftazidime disks alone, and in combination with clavulanic acid. An organism is interpreted as containing ESBL if there is an increase in zone size greater than or equal to 5 mm, comparing the combination disk to cephalosporin alone.[9] Although scarce, the published studies do indicate that the incidence of peritonitis in CAPD patients due to ESBL-producing Enterobacteriaceae is on the rise.[6] This seems to be true even in our hospital, where over the last year, four cases of ESBL peritonitis have been isolated out of a total of 140 PD fluid cultures. Although this is still small in number, there has been a definite rise when compared to its incidence in previous years. Fortunately, the index case has been the only pediatric case that we have encountered. Apart from this increasing trend in the occurrence of ESBL, these studies have also demonstrated a higher incidence of treatment failure and death in this group when compared to the non-ESBL group.[6] Increased adverse results might be because of a delay in the initiation of appropriate antibiotic therapy, secondary to problems in detecting the ESBL-producing strains. In addition, the ESBL strains are also often co-resistant to other classes of antibiotics such as fluroquinolones and aminoglycosides, making treatment challenging.[10] Uses of cephalosporin and gastric acid inhibitors have been associated with ESBL.[6] Our case did have both the risk factors, as he had been given cephalosporins twice over the last 3 months for suspected sepsis, and was also on a regular proton pump inhibitor for ‘heartburn’. It has been shown that prolonged gastric acid inhibition results in intestinal bacterial overgrowth,[11] and hence, although not definitely proven, this may explain the increased incidence of ESBL peritonitis, particularly among those also being exposed to cephalosporins.
Intravenous meropenem is usually the drug of choice for systemic ESBLs, but the picture is not clear for ESBL peritonitis. Parturi et al. documented failure in the resolution of ESBL KP peritonitis, despite intravenous meropenem as well as removal of the PD catheter.[7] In contrast, we did achieve a good response with intravenous meropenem, but we combined it with oral cotrimoxazole. Localized treatment, that is, IP antibiotic, is the recommendation for peritonitis among patients on PD.[1] Unfortunately, similar to our case, most of the IP antibiotics recommended by the ISPD guideline are often found to be resistant to ESBL, and IP use of Meropenem is not mentioned in the guideline. A single case report is available on the use of IP flomoxef (a synthesized oxacephem) in ESBL peritonitis, in an adult on CAPD.[12] Due to the absence of strong evidence as well as it not being readily available, we did not use it in our case. A recent case report has documented success in the treatment of Enterobacter aerogenes infected diabetic foot with intra-peritoneal meropenem, which achieved an excellent blood level.[13] This success needs to be studied in a scenario of ESBL peritonitis, before it can be recommended.
The increasing report of ESBL-producing Enterobacteriaceae highlights the urgent need for studies exploring the role of IP antibiotic-like meropenem vis-à-vis parenteral therapy, as well as the need for a revision of the ISPD recommendations, incorporating suggestions for ESBL peritonitis. Finally, one has to remember that judicious use of antibiotics and infection prevention measures like hand wash and proper exit site care will still be the most efficacious step in our fight against these emerging multiple antibiotic-resistant bacteria.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393-423.
- [Google Scholar]
- Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumonia and Serratia marcescens. Infection. 1983;11:315-1.
- [Google Scholar]
- Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008;13:Pii:19044.
- [Google Scholar]
- Incidence of ESBL producers amongst Gram-negative bacilli isolated from intra-abdominal infections across India (based on SMART study, 2007 data) J Assoc Physicians India. 2011;59:287-92.
- [Google Scholar]
- Community acquisition of ESBL-producing Escherichia coli: A growing concern. Med J Aust. 2009;190:45-6.
- [Google Scholar]
- Risk factors and outcomes of extended-spectrum beta-lactamase-producing E. coli peritonitis in CAPD patients. Perit Dial Int. 2006;26:191-7.
- [Google Scholar]
- Extended spectrum beta-lactamase-producing Klebsiella pneumoniae chronic ambulatory peritoneal dialysis peritonitis treated successfully with polymyxin B. Heart Lung. 2005;34:360-3.
- [Google Scholar]
- Escherichia coli peritonitis in patient undergoing peritoneal dialysis: A serious problem that may get worse. Perit Dial Int. 2006;26:174-7.
- [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement M100-S15. Wayne, PA: Clinical and Laboratory Standards Institute, formerly NCCLS; 2005.
- [Google Scholar]
- Extended-spectrum beta-lactamases: Implications for the clinical microbiology laboratory, therapy, and infection control. J Infect. 2003;47:273-95.
- [Google Scholar]
- Risk factors for developing peritonitis caused by micro-organisms of enteral origin in peritoneal dialysis patients. Perit Dial Int. 1998;18:41-5.
- [Google Scholar]
- Expanded-spectrum beta-lactamase producing Klebsiella pneumoniae-related peritonitis in a patient on peritoneal dialysis. Am J Kidney Dis. 2004;44:e102-6.
- [Google Scholar]
- Intraperitoneal Meropenem administration: A possible alternative to the intravenous Route. Perit Dial Int. 2010;30:250-1.
- [Google Scholar]







