Translate this page into:
The short-term impact of protocol biopsies in a live-related renal transplant program using tacrolimus based immunosuppression
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The aim of the study was to assess the impact of protocol biopsies in a live-related renal transplant program using tacrolimus-based immunosuppression in the short term. Eighty-three live-related transplant recipients were randomly allocated to protocol biopsy group (Group I, n = 40) and a control group (Group II, n = 43). Other immunosuppressants in these groups consisted of either mycophenolate mofetil or azathioprine and steroids. Protocol biopsies were conducted in biopsy group at 1, 6, and 12 months post-transplant. The non-biopsy group was followed by serial serum creatinine and biopsies in them were conducted as and when clinically indicated. Both groups were analyzed at 12 months with respect to graft function and survival. The two groups were similar with respect to age, number of dialysis pre-operatively, tacrolimus levels, induction therapy, donor age, and donor glomerular filtration rate. Forty protocol biopsies were conducted at 1 month, 31 at 6 months, and 26 at 12 months. The prevalence of sub-clinical rejection at 1, 6, and 12 months in these biopsies was 17.5%, 11.2%, and 10.3%, respectively. The prevalence of calcineurin inhibitor toxicity during same period was 15%, 15.5%, and 14.4%, respectively. The cumulative rejection rate in Group I and Group II at 12-month follow-up was 10.3% and 11.3% (P = 0.78), respectively, and cumulative calcineurin inhibitor toxicity at 12 months was 14.4% and 9.3% (P = 0.59), respectively, were not statistically significant. There was no difference in graft survival and function at 1 year. Protocol biopsies have a limited role in a well-matched renal transplant program with tacrolimus-based immunosuppression in the short term. However, the long-term impact of protocol biopsies needs further evaluation.
Keywords
Calcineurin inhibitors
live-related renal transplantation
protocol renal biopsy
sub-clinical rejection
Introduction
Renal transplantation is the treatment of choice for end-stage renal disease.[1] The focus of interest is to increase the life of the transplanted graft. The introduction of cyclosporine in early 1980s and tacrolimus in early 1990s has decreased the incidence of acute rejection episodes, but it has not been translated into improved long-term graft survival. Pascual, et al, reported the annual rate of graft loss at 3-5%. They found chronic rejection and death with a functioning graft as leading cause for this graft loss.[2]
The development of chronic rejection has been consistently correlated with acute rejection episodes. But since the long-term survival has not improved with concomitant decline in episodes of acute rejection, the focus has shifted to know the prevalence and pathological significance of sub-clinical allograft inflammation.[3] It has been shown that treatment of clinically silent tubulitis lead to a significant improvement in renal function and improved long-term graft survival.[34]
Serum creatinine has been shown to be a relative insensitive marker for detection of early graft pathology and is considered unreliable for assessment of adequacy of immunosuppression.[56] Indeed, it has been shown that the features of chronic allograft nephropathy are reversible only within first 12 weeks post-transplant.[7]
The introduction of tacrolimus in early 1990s led to various studies comparing it with cyclosporine. These studies have consistently shown tacrolimus to be superior to cyclosporine.[58910]
In this single center trial, the impact of protocol biopsies in the short term in a live-related renal transplant program using tacrolimus-based immunosuppression was determined.
Materials and Methods
Patient selection
The study was a single center randomized prospective longitudinal study involving recipients of live-related renal transplant. Ethical clearance was taken from the hospital ethical committee. All patients were informed that they do not have to participate and were informed the risks and benefits of protocol biopsies. They were also explained that they could opt out at any stage. Patients who underwent a live-related renal transplant from April 2006 to May 2007 were enrolled. Patients with stable graft function having a serum creatinine of <1.9 mg/dl with a normal ultrasound and diethylenetriamine penta-acetic acid (DTPA) scan at 1 week post-transplant were included in the study. In addition, patients had to have no clotting abnormalities and have tacrolimus levels within the therapeutic range. All patients undergoing a second renal transplant were excluded from the study. There was no difference between the two groups as regards body mass index and lipid profile at 1 week and 1 year post-transplantation.
Immunosuppression
The immunosuppression was started on the day before transplantation. Tacrolimus was given in dose of 0.15 mg/kg along with either mycophenolate mofetil in dose of 500 mg twice a day or azathioprine in dose of 1.5-2 mg/kg. Patients who could afford were put on mycophenolate mofetil and those who could not were put on azathioprine. The dose of tacrolimus was adjusted to keep trough levels at 10-12 ng/ml in first 3 months, 8-10 ng/ml in the next 3 months, and 5-8 ng/ml thereafter. Tacrolimus levels were determined by the Abbot IMx tacrolimus II assay (Abbot Laboratories, Abbot Park, IL, USA). This procedure is based on the micro-particle enzyme immunoassay technology. All patients received methylprednisolone, in the morning of surgery pre-operatively and evening of surgery post-operatively. Oral prednisolone was started on first post-operative day at a dose of 20 mg once a day. It was tapered to 10 mg at the end of 6 months and 7.5 mg at the end of 12 months. Induction therapy with an IL-2 inhibitor was given to all patients who could afford this therapy. Daclizumab induction was used in 9 patients in the biopsy group and 12 in the non-biopsy group. Since this sample size was not sufficient, the impact of induction therapy was not analyzed.
Biopsy protocol
The patients included in the biopsy group were biopsied at 1, 6, and 12 months and as and when clinically indicated. The patients who underwent biopsy when clinically indicated were not again biopsied at the time required in the protocol, if it was done within 1 month of time of protocol biopsy. But they continued to be part of the biopsy group. The results of this biopsy were excluded from protocol biopsy for analysis. Crossover to other treatment regimen was considered as endpoint. Specimens were taken under ultrasound guidance from upper pole of the transplanted kidney under local anesthesia using 18-gauge spring loaded automated punch biopsy gun. Specimens reported as inadequate were re-biopsied.
Analysis of biopsy specimen
The specimens were fixed in formalin. They were analyzed by a single consultant pathologist who was blinded with respect to nature of immunosuppression. The Banff criteria[11] were followed for reporting. Since the study commenced in 2006, the Banff criteria of 1999 were used. The state of glomeruli, tubules, blood vessels, and interstitium were analyzed and abnormal histology if any was reported. Opinion on the single most probable cause for the given histological picture was taken with respect to the various causes of graft dysfunction which are as follows:
-
Acute rejection,
-
Calcineurin inhibitor toxicity,
-
Acute tubular necrosis, and
-
Chronic allograft nephropathy.
The pathologist was asked to report on the single most probable diagnosis. C4d was not done routinely as facilities were not available for the same. Recurrent disease or de novo glomerulonephritis was not seen in any of the biopsies in any group.
Anti-rejection therapy
The study was conducted with “intent to treat.” Sub-clinical rejection as stated by Rush, et al., required an acute inflammatory score of ≥4 (≥ Grade I) in protocol biopsy and an increase in serum creatinine of <10% from the defined baseline.[4] All patients with Grades I-III were given anti-rejection therapy in the form of three doses of injection, methyl prednisolone 500 mg over 3 days.
Follow-up
The patients were followed by serial creatinine measurements. They were subjected to biopsy at 1, 6, and 12 months. Patients who had acute rise in serum creatinine (>0.3 mg/dl) were biopsied as and when indicated.
Dose of tacrolimus was adjusted considering both the level and the results of protocol biopsy. Estimated glomerular filtration rate for the patients was calculated at 1, 6, and 12 months using modified diet for renal disease (MDRD) formula.[12]
The patients in the non-biopsy group were followed by serial serum creatinine. Their dose of immunosuppression was adjusted based on the level of drugs. They were subjected to biopsy as and when clinically indicated, i.e., a rise in serum creatinine of >0.3 mg/dl in the presence of normal therapeutic drug levels. Estimated glomerular filtration rate was calculated at 1, 6, and 12 months using MDRD.
Statistical analysis
The data were stored in Microsoft excel worksheet. Chi-square test and Fisher test were used for analysis of non-categorical data such as sex and donor profile. Repeated measure of analysis was used to compare the estimated glomerular filtration rate. Independent t-test was used for categorical data. Analysis of variance was used for analysis of categorical data between various groups. All analysis was done using service provisioning system software (SPSS), version 11.6.
Results
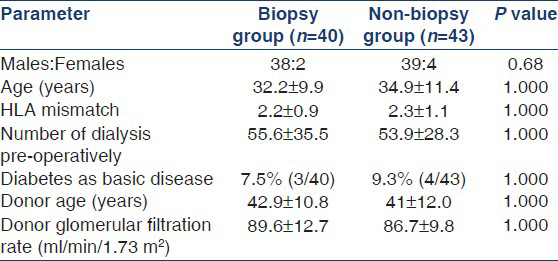
A total of 83 patients were randomized with 40 patients in protocol biopsy group and 43 in non-biopsy group. The demographic profile of the patient is given in Table 1. The two groups were similar with respect to age, number of HLA mismatches, and number of dialysis y. There was no statistical difference between the two groups with respect to donor age and donor glomerular filtration rate. The two groups were similar with respect to patients on mycophenolate mofetil and azathioprine. The non-biopsy group had more patients receiving induction therapy but this was not statistically significant. The mean tacrolimus level was also not statistically different between two groups.
Protocol biopsy group
At 1 month, 40 patients underwent protocol biopsies. All patients having sub-clinical rejection received methyl prednisolone pulse therapy. The patients showing calcineurin inhibitor toxicity had their dose of tacrolimus reduced. The dose was reduced to achieve the levels toward the lower limit of normal value as per standard protocol.
At 6-month follow-up, 2 out of 40 (5%) patients in protocol biopsies were converted to other immunosuppression. One of the patients was converted to everolimus as the patient had biopsy features suggestive of calcineurin inhibitor toxicity and had persistently high levels of tacrolimus despite lowering the dose. The other patient was converted to cyclosporine due to failure to achieve adequate levels of tacrolimus. There was no clinically indicated biopsy. Out of the possible 38 protocol biopsies, 31 were conducted. In seven patients, protocol biopsies were not conducted, six patients did not turn up at required time, and one patient had high sugars requiring admission at the time of protocol biopsy. The patients in whom protocol biopsies were not conducted were not included in the analysis.
At 12-month follow-up, there were no clinically indicated biopsies, however; 26 protocol biopsies were conducted. The remaining 14 patients did not turn for up for the biopsy. These 14 patients were not included in the analysis of data to avoid any confusion.
Non-biopsy group
The non-biopsy group was followed by serial creatinine. The results of biopsies are shown in Table 2. At 6-month follow-up, one patient was changed over to cyclosporine as serum creatinine was in the range of 1.8-2.1 mg/dl despite persistently high levels of tacrolimus. The patient had a drop in serum creatinine reaching range of 1-1.2 mg/dl after change over to cyclosporine.

At 12-month follow-up, there was no graft loss or patient death.
Sub-clinical/acute rejection
The prevalence of sub-clinical rejection in the protocol biopsy group at 1, 6, and 12 months in these biopsies was 17.5%, 3.2%, and 7.7%, respectively. The incidence of acute rejection in first month post-transplant was 4.6%. The incidence between 1 and 6 months was 6.9% and there was no new acute rejection episode between 6 and 12 months. The cumulative rejection frequency at 12 months in biopsy group was 10.3%, whereas in non-biopsy group, the incidence of acute rejection episodes was 11.3% but the difference was not statistically significant as shown in Table 3.

Calcineurin inhibitor toxicity
The prevalence of biopsy features suggestive of calcineurin inhibitor toxicity at 1, 6, and 12 months protocol biopsy was 17.5%, 11.2%, and 10.3%, respectively. There was no case of calcineurin inhibitor toxicity at 1 month. There were two new cases of calcineurin inhibitor toxicity between 1 and 6 months and 6 and 12 months. There was no statistical significant difference in cumulative calcineurin inhibitor toxicity at 12 months as shown in Table 4.

Graft function and survival
There was no graft loss in either of two groups. The mean glomerular filtration rate at 1 year in protocol biopsy group was 74.8 ± 16.9 ml/min/1.73 m2, whereas in non-biopsy group was 73.9 ± 15.8 ml/min/1.73 m2, P = non-significant. Thus, there was no difference in graft function in two groups at 1 year [Table 5].

There was no difference in infection episodes between the two groups.
Complications of biopsy
Four patients had gross hematuria. All of them resolved on conservative management without need for any blood transfusion. There was no graft loss.
Discussion
The study was done to assess the impact of protocol biopsy on graft function on patients on tacrolimus-based immunosuppression in live-related renal transplant recipients. The study showed that there may not be any benefit of protocol biopsy on graft function in short term.
The cumulative rejection frequencies in protocol biopsy group in our study at 1, 6, and 12 months were 17.5%, 11.2%, and 10.3%, respectively. Solez, et al,[9] in their randomized study, had reported the rate of rejection at 1 year in patients on tacrolimus to be 32.9%. The higher rate of rejection in their study may be due to the fact that the study had cadaveric renal transplant recipients and used azathioprine as second immunosuppressant. Nankivell, et al,[5] had reported that mycophenolate mofetil reduced tubulitis in graft kidney and was more effective than azathioprine in reducing sub-clinical rejection.
Gloor, et al,[13] had reported prevalence of sub-clinical rejection to be 2.4% at 3-month surveillance biopsies in live-related transplant in patients on tacrolimus and mycophenolate mofetil (1 g twice daily). The low rate of sub-clinical rejection could be possibly due to higher dose of mycophenolate mofetil than used in our study. Also our study included patients who were on azathioprine.
Moreso, et al,[14] had reported the prevalence of sub-clinical rejection in patients on tacrolimus and mycophenolate mofetil to be 14.2% biopsied between 4 and 6 months. The prevalence reported by Rowshani, et al,[15] on similar treatment at 6 months was 15.2%. These rates were similar to our study.
Rush, et al., in their multicenter randomized study, had shown that there is no benefit of protocol biopsies in patients on tacrolimus- and mycophenolate mofetil-based immunosuppression. They reported the prevalence of sub-clinical rejection at 6 months was 9% in their study. Creatinine clearance in their study at 6 months was 72.9 ± 21.7 ml/min in biopsy arm and 68.9 ± 18.35 ml/min in control arm (P = 0.18) which was not statistically significant.[16]
In our study, the prevalence of calcineurin inhibitor toxicity was more than sub-clinical rejection, 14.4% versus 10.3%, respectively. Solez, et al. had reported the prevalence of tacrolimus toxicity to be 24.1% at 2-year protocol biopsy. They had also shown that nephrotoxicity and acute rejection were significant predictors of chronic graft nephropathy at 2 years after transplantation. To assess the impact of this on graft function requires a longer follow-up.
In conclusion, protocol biopsies may have a limited role in patients on tacrolimus in well-matched live-related renal transplant program role as far as detection of sub-clinical rejection and its impact on graft function are concerned. However, it may be an important tool to assess toxicity of calcineurin inhibitors as suggested by Racusen.[17] Ekberg, et al,[18] had recently reported that recipients on low-dose tacrolimus have better graft function as compared to standard dose cyclosporine and low-dose cyclosporine and sirolimus. Thus, protocol biopsy may be required to assess the best possible dose of tacrolimus without compromising graft function.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605-12.
- [Google Scholar]
- Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346:580-90.
- [Google Scholar]
- Beneficial effects of treatment of early subclinical rejection: A randomized study. J Am Soc Nephrol. 1998;9:2129-34.
- [Google Scholar]
- Significance of tubulitis in chronic allograft nephropathy: A clinicopathologic study. Clin Transplant. 1997;11:139-41.
- [Google Scholar]
- Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation. 2004;78:242-9.
- [Google Scholar]
- Protocol transplant biopsies: An underutilized tool in kidney transplantation. Clin J Am Soc Nephrol. 2006;1:138-43.
- [Google Scholar]
- Reversibility of chronic renal allograft rejection. Critical effect of time after transplantation suggests both host immune dependent and independent phases of progressive injury. Transplantation. 1994;58:93-9.
- [Google Scholar]
- Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: A report of the European Tacrolimus Multicenter Renal Study Group. Transplantation. 1997;64:436-43.
- [Google Scholar]
- Histopathologic findings from 2-year protocol biopsies from a U.S. multicenter kidney transplant trial comparing tarolimus versus cyclosporine: A report of the FK506 Kidney Transplant Study Group. Transplantation. 1998;66:1736-40.
- [Google Scholar]
- A long-term comparison of tacrolimus (FK506) and cyclosporine in kidney transplantation: Evidence for improved allograft survival at five years. Transplantation. 2002;73:775-82.
- [Google Scholar]
- The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713-23.
- [Google Scholar]
- MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant. 2005;5:1306-11.
- [Google Scholar]
- Subclinical rejection in tacrolimus-treated renal transplant recipients. Transplantation. 2002;73:1965-8.
- [Google Scholar]
- Baseline immunosuppression is associated with histological findings in early protocol biopsies. Transplantation. 2004;78:1064-8.
- [Google Scholar]
- No difference in degree of interstitial Sirius red-stained area in serial biopsies from area under concentration-over-time curves-guided cyclosporine versus tacrolimus-treated renal transplant recipients at one year. J Am Soc Nephrol. 2006;17:305-12.
- [Google Scholar]
- Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: A randomized study. Am J Transplant. 2007;7:2538-45.
- [Google Scholar]
- Protocol transplant biopsies in kidney allografts: Why and when are they indicated? Clin J Am Soc Nephrol. 2006;1:144-7.
- [Google Scholar]
- Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562-75.
- [Google Scholar]







