Translate this page into:
Melatonin improves sleep quality in hemodialysis patients
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Disturbed sleep is common in end-stage renal disease (ESRD). Exogenous melatonin has somniferous properties in normal subjects and can improve sleep quality (SQ) in several clinical conditions. Recent studies have shown that melatonin may play a role in improving sleep in patients undergoing dialysis. The goal of the present study was to assess the effect of exogenous melatonin administration on SQ improvement in daytime hemodialysis patients. Lipid profile and the required dose of erythropoietin (EPO) are also reported as secondary outcomes. In a 6-week randomized, double-blind cross-over clinical trial, 3 mg melatonin or placebo was administered to 68 patients at bedtime. A 72-h washout preceded the switch from melatonin to placebo, or vice versa. SQ was assessed by the Pittsburgh sleep quality index (PSQI). Sixty-eight patients completed the study protocol and were included in the final analysis. Melatonin treatment significantly improved the global PSQI scores (P < 0.001), particularly subjective SQ (P < 0.001), sleep efficiency (P = 0.005) and sleep duration (P < 0.001). No differences in sleep latency and daytime sleepiness were observed. Melatonin also increased the high-density lipoprotein (HDL) cholesterol (P = 0.003). The need for EPO prescription decreased after melatonin treatment (P < 0.001). We conclude that melatonin can improve sleep in ESRD. The modest increase in HDL cholesterol and decrease in the EPO requirement are other benefits associated with this treatment
Keywords
End-stage renal disease
hemodialysis
melatonin
pittsburgh sleep quality index
sleep
Introduction
Poor sleep quality (SQ) is common among patients on maintenance hemodialysis (HD).[123] SQ is important not only as a predictor of quality of life but also as a mortality risk among these patients.[45] Evidence is not conclusive about the source of sleep disturbance in these patients. However, absence of a nocturnal rise in melatonin concentration in daytime HD patients has been documented,[6] suggesting that it may be related to the degree of chronic kidney disease.[7] In contrast, some studies on HD patients have shown a relationship between supra-physiological concentrations of melatonin and its major metabolite, 6-sulfatoxymelatonin, with a diurnal rhythm in spite of insomnia, delayed sleep onset, night-time arousals and restless-leg syndrome.[8] Also, a small non-randomized trial showed significant improvements in subjective and objective sleep parameters as well as partial recovery of nocturnal melatonin rhythm after switching to in-center nocturnal HD.[9] However, other studies have shown that despite recovery of the melatonin rhythm after nocturnal HD, patients still suffered from poor sleep.[10]
Recent studies have underlined the benefit of exogenous administration of melatonin in sleep disorders in many conditions such as old adult insomnic,[11] parkisonism,[12] asthma,[13] chronic obstructive pulmonary disease (COPD)[1415] and HD patients.[16] Also, there is a promising prospect that administration of melatonin could improve anti-oxidative defence and the lipid profile.[171819]
The primary aim of this study is to investigate the efficacy of oral melatonin in improving quality of sleep in our patients on conventional daytime HD. In addition, lipid profile changes and erythropoietin (EPO) dose required after melatonin treatment are also evaluated.
Materials and Methods
Study design and protocol
This was a randomized, double-blind cross-over clinical trial [Figure 1] to evaluate the effectiveness of melatonin versus placeboin improving SQ in HD patients. The research project was approved by the Human Research Ethics Committee of the Arak University of Medical Sciences in Iran and was registered at Iranian Registry of Clinical Trials with the registration number IRCT 201108077253N1.
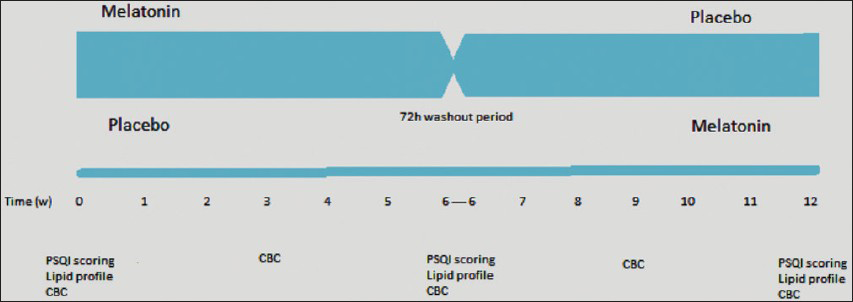
- Study design
Patients were screened for inclusion in the study by their SQ score base on the Pittsburgh Sleep Quality Index (PSQI).[20] Inclusion criteria were age >18 years, ability to give informed consent, duration of HD >3 months, PSQI score ≥5 and adherence to regular and steady dialysis program or medication that interfere with melatonin secretion (such as beta adreno-receptors blocking drug). Exclusion criteria included known major illness (malignancy, active infection and uncontrolled heart failure), pregnancy, iron deficiency anemia, poor control diabetes mellitus (hemoglobin A1c >7.5), current use of melatonin or known allergy of melatonin, acute medical or surgical condition that required hospitalization or operation throughout the study and dementia or psychotic disorder as diagnosed by researchers that interferes with patient's participation in this trial. All patients received daytime dialysis, and the total hours per week varied from 8 to 11.5 h.
One hundred and nineteen out of 163 patients consented to fill out the PSQI questionnaire. Eighty-seven cases (73%) had PSQI score ≥5. Finally, 82 patients who met the inclusion criteria were enrolled in this trial. Patients were randomly assigned to placebo or melatonin for 6 weeks. Melatonin (Melatonin Plus) (Schiff®, Salt Lake City, USA) and placebo were packaged in identical 3-mg tablets. Melatonin plus has added Theanine, a natural compound found in green tea and vitamin B6 (10 mg). Dosage was set as one tablet taken at bedtime. After 6 weeks, patients underwent a 3-day washout period, followed by 6 weeks of the alternative therapy (from melatonin to placebo, and vice versa). Compliance was confirmed by pill count.
Measurement of sleep quality
SQ was measured using the PSQI. This 19-question questionnaire measures the SQ for the previous month. This questionnaire assesses seven components (each scored from 0 to 3) of subjective SQ, sleep efficiency, sleep disturbance, sleep latency, sleep duration, use of sleep medications and daytime dysfunction. The global PSQI score is calculated over a range of values from 0 to 21 by adding up these seven component scores. A patient with a global PSQI score ≥5 was considered to be a bad sleeper. In this study, patients completed this questionnaire by themselves or received assistance from research nurses during HD. Patients completed the PSQI questionnaire at the beginning of study and after the 6 weeks treatment by drug or placebo. The initial PSQI questionnaire score (W0) was used to determine eligibility to participate in the study (PSQI score ≥5).
Laboratory methods
Blood samples were collected prior to the HD session. Samples for complete blood count (CBC) were collected every 3 weeks and for total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol were collected at the beginning and at the end of the study in each arm.
EPO (PooyeshDarou-Poetin®, Tehran, Iran) was injected subcutaneously. Serum hemoglobin were measured every 3 weeks and used for EPO dose adjustment. Dose adjustment was done base on the patient's hemoglobin (Hgb) level, the target Hgb level, the observed rate of increase in Hgb level and the clinical circumstances, as recommended by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines.[21] Injected EPO value was assessed by reviewing the dialysis charts and asking from patients. Cumulative prescribed EPO within 6 weeks, and also required dose at the end of 6 weeks of treatment by drug/placebo, were used to compare the drug/placebo effects in each arm.
Statistical analyses
Statistical analysis was performed using Statistical Package for the Social Sciences SPSS 17.0 and two-sample paired-group t test. The primary end point was the change in PSQI scores at the end of 6 weeks treatment of melatonin or placebo. Pre-defined secondary end points were the change in lipid profile parameters (HDL cholesterol, LDL cholesterol, triglyceride), hemoglobin and EPO dose required in proportional of hemoglobin in each arm. Upon completion of the study, primary and secondary end points were evaluated for all patients using a 95% significance criterion (P < 0.05).
Results
Demographic data
Eighty-two consenting patients randomly took placebo or melatonin. During the course of the study, a total of 14 patients were withdrawn for reasons explained in Table 1. Sixty-eight patients (53% men; mean age of 58 ± 14 years; vintage of 6-296 months and 43% diabetics) remained in the study until completion. The majority of the subjects received HD for 4 h, three times a week. Baseline patient characteristics are shown in Table 2.


Baseline global and component PSQI scores and lab results
The baseline, mean global PSQI score of the patients was 10.58 ± 3.44 (95% Confidence Interval, 9.19-11.97). The baseline lipid profile and hemoglobin value of the sample is shown in [Table 2].
Effect of melatonin on sleep
PSQI scores were calculated at the end of 6 weeks. Significant improvements in global scores were observed in the melatonin-treated arm. Also, there was a statistically significant drop in the scores of sleep duration, sleep disturbance and sleep efficiency. In spite of improvement of subjective SQ, there was no improvement in daytime dysfunction after drug treatment. There was no meaningful difference in need for hypnotic sleep medications between groups. The global PSQI score and its components at the end of treatment are shown in Table 3. No significant differences were observed in PSQI scores by age, gender, time on HD or presence of diabetes (P > 0.05) (data not shown). There were also no serious detectable side-effects after melatonin treatment.

Effect of melatonin on lipid profile
There was no change in serum total cholesterol, LDL cholesterol and serum triglyceride levels throughout the study period [Table 4]. However, compared with the placebo, melatonin increased HDL cholesterol at the end of week 6 (P = 0.003).

Effect of melatonin on hematocrit and EPO dosage
There was no significant change in hemoglobin levels throughout the study period in both the drug treated and the placebo group [Table 5]. However, given the hemoglobin level, melatonin treatment decreased EPO dosage requirement.

There were also no statistically significant changes in either serum phosphorous, calcium-phosphorous product, serum parathyroid hormone or ferritin levels in both groups (P > 0.05) (data not shown).
Discussion
Melatonin is found widely in nature, ranging from unicellular organisms, plants and fungi to animals and humans.[22] In the human body, melatonin is secreted nocturnally by the pineal gland as an endogenous sleep regulator.[22] Various studies have supported the effectiveness of exogenous melatonin in sleep disorders,[12131415] insomnia,[1119] metabolic syndrome,[23] blood pressure regulation,[182425] neurodegenerative diseases[11] and disorders of oxidative damage.[1718] As complaints on poor quality of sleep are common among HD patients, we proposed to examine the effect of endogenous melatonin on SQ in these patients. In a meta-analysis, Brzezinski et al.,[26] using information derived from 17 different studies (involving 284 subjects) studied sleep-onset latency, total sleep duration and sleep efficiency. They reported that melatonin treatment can significantly improve these sleep components. However, the magnitude of this effect appears to be clinically insignificant.
Another meta-analysis by Braam et al.,[27] used nine different studies including a total of 183 individuals with intellectual disabilities. The study concluded that melatonin was effective in increasing the total sleep time and reducing the sleep latency and the number of wakes per night in patients.
In contrast, meta-analysis of the effects of melatonin in sleep disturbances, including all age groups (six randomized controlled trials with 97 participants) conducted by Buscemi et al.,[28] failed to document significant and clinically meaningful effects of exogenous melatonin on SQ, efficiency or latency. According to this analysis, melatonin did not have any effect on sleep-onset latency and sleep efficiency, SQ, wakefulness after sleep onset and the total sleep time in people with primary insomnia.
A recent study by Koch et al.,[16] suggests that melatonin may lead to an improvement of subjective and objective sleep parameters in HD patients. Using the sleep questionnaire and actigraphy, the study assessed the effect of melatonin (6 weeks melatonin 3 mg) on SQ in 20 HD patients. It concluded that melatonin treatment can significantly improve objective sleep-onset latency, sleep efficiency and actual sleep time.
Our survey was similar to that of Koch et al.,[16] except that we used only the PSQI questionnaire for assessment of SQ with a much larger sample size. Our data showed that 6-week melatonin administration improved patient-reported quality of sleep, particularly in terms of duration, disturbance and efficiency, without any impact on sleep latency and daytime dysfunction. Also, treatment with melatonin did not decrease the post-treatment need for sleep-inducing medication or hypnotics. Our results are different from those of Koch et al.,[16] where they have shown a significant improvement in sleep latency. Most studies on the effect of melatonin on SQ have failed to show any profound improvement on daytime sleepiness except the studies by Garfinkel et al.,[19] and Lemoin et al.[11] Both these studies stress the positive effect of melatonin on decreasing daytime sleepiness. Our results do not indicate any improvement in daytime dysfunction as reported by patients. Our results corroborate the findings by Nunes et al.,[14] indicating no change in daytime sleepiness despite significant improvement in global PSQI scores, sleep latency and sleep duration in COPD. In our view, the differences in findings in various studies could be explained by the variations in the duration, administered dose, sample size, heterogeneity of the selected samples and the way in which outcomes were measured.
In our study, we also monitored the lipid profile of patients after 6 weeks of melatonin or placebo prescription. Our data demonstrated significant increases in HDL cholesterol levels (P < 0.003) after melatonin treatment. In contrast, Garfinkel et al.,[19] showed no significant changes in lipid profile after 3 weeks of melatonin (2 mg) treatment in 36 type 2 diabetic patients with insomnia. Meanwhile, Koziróg et al.,[18] suggest that melatonin can decrease the LDL cholesterol without any change in HDL cholesterol in patients with metabolic syndrome.
The significant decrease in EPO dose requirements is another positive outcome associated with melatonin use in HD patients. We were unable to find any references in regards to this finding in the published literature. However, a longer follow-up period and a higher number of patients are required to really obtain data with a higher statistical power. We speculate that our results may in part be due to the anti-oxidative effect of melatonin. Herrera et al.,[17] have shown that oral consumption of melatonin can prevent oxidative stress of intravenous iron and EPO, both commonly used to treat anemia in chronic HD patients. Karimungi et al.,[29] demonstrated diurnal sensitivity in melatonin-induced hematological changes in the male albino rat. They concluded that melatonin may have a modulatory role in hematopoiesis and its rhythms.
In our study, we also attempted to control for known potential confounding variables for quality of sleep, such as major comorbidity, overt mood disorders and changes in treatment protocol, which could typically induce major changes in the target biochemical parameters (glucose, phosphorous, etc.,) However, several limitations of this report should be noted. The main limitation of this study is the absence of polysomniography or actigraphy, without which it is not possible to ascertain the exact cause of insomnia and sleep disturbance.[30] Also, subjective reports of SQ do not have a single criterion standard.
The results from the present study, showing sleep improvement and possible beneficial effect on hematopoiesis, are promising. However, this study had a relatively small sample size and a short investigation period. A longer-term multicenter study with a larger sample will be needed to further confirm the benefits of exogenous melatonin in HD patients in the future.
Conclusions
Our study suggests that melatonin may emerge as a safe, low-cost therapy for improving SQ in HD patients. The modest increase in HDL value and a decrease in the required EPO dose could be the other benefits of this treatment.
Acknowledgment
The authors deem it is necessary to offer their thanks and appreciation to the Vice Chancellor for Research of the Arak University of Medical Sciences for providing the financial and logistical resources. They would also like to thank the very generous staff at Vali-Asr Hospital and the patients who participated in this clinical trial and gave them tremendous help and support in this study.
Source of Support: The Vice Chancellor for Research of the Arak University of Medical Sciences
Conflict of Interest: None declared.
References
- Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant. 2006;21:184-90.
- [Google Scholar]
- Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transplant. 2009;24:247-51.
- [Google Scholar]
- Quality of sleep and health-related quality of life in haemodialysis patients. Nephrol Dial Transplant. 2003;18:126-32.
- [Google Scholar]
- Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23:998-1004.
- [Google Scholar]
- Impairment of endogenous melatonin rhythm is related to the degree of chronic kidney disease (CREAM study) Nephrol Dial Transplant. 2010;25:513-9.
- [Google Scholar]
- Effects of nocturnal hemodialysis on melatonin rhythm and sleep-wake behavior: An uncontrolled trial. Am J Kidney Dis. 2009;53:658-64.
- [Google Scholar]
- Different melatonin rhythms and sleep-wake rhythms in patients on peritoneal dialysis, daytime hemodialysis and nocturnal hemodialysis. Sleep Med. 2010;11:242-6.
- [Google Scholar]
- Clearance of melatonin and 6-sulfatoxymelatonin by hemodialysis in patients with end-stage renal disease. J Pineal Res. 2001;31:222-7.
- [Google Scholar]
- Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res. 2007;16:372-80.
- [Google Scholar]
- Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson's disease. A randomized, double blind, placebo-controlled study. J Neurol. 2007;254:459-64.
- [Google Scholar]
- Melatonin improves sleep in asthma: A randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2004;170:947-51.
- [Google Scholar]
- Effect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary disease. Braz J Med Biol Res. 2008;41:926-31.
- [Google Scholar]
- Effect of melatonin on sleep quality of COPD intensive care patients: A pilot study. Chronobiol Int. 2000;17:71-6.
- [Google Scholar]
- The effects of melatonin on sleep-wake rhythm of daytime haemodialysis patients: A randomized, placebo-controlled, cross-over study (EMSCAP study) Br J Clin Pharmacol. 2009;67:68-75.
- [Google Scholar]
- Melatonin prevents oxidative stress resulting from iron and erythropoietin administration. Am J Kidney Dis. 2001;37:750-7.
- [Google Scholar]
- Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011;50:261-6.
- [Google Scholar]
- Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: A randomized, double-blind, crossover study. Diabetes Metab Syndr Obes. 2011;4:307-13.
- [Google Scholar]
- The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213.
- [Google Scholar]
- KDOQI, National Kidney Foudnation. II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47(Suppl 3):S16-85.
- [Google Scholar]
- Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004;25:177-95.
- [Google Scholar]
- Melatonin and the metabolic syndrome: Physiopathologic and therapeutical implications. Neuroendocrinology. 2011;93:133-42.
- [Google Scholar]
- Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43:192-7.
- [Google Scholar]
- Effects of melatonin on vascular reactivity, catecholamine levels, and blood pressure in healthy men. Am J Cardiol. 1999;83:1417-9.
- [Google Scholar]
- Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med Rev. 2005;9:41-50.
- [Google Scholar]
- Exogenous melatonin for sleep problems in individuals with intellectual disability: A meta-analysis. Dev Med Child Neurol. 2009;51:340-9.
- [Google Scholar]
- Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: Meta-analysis. BMJ. 2006;332:385-93.
- [Google Scholar]
- Diurnal sensitivity in melatonin-induced hematological changes in the male albino rat. Biol Signals. 1996;5:283-90.
- [Google Scholar]
- Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:519-29.
- [Google Scholar]







