Translate this page into:
Oxalate nephropathy: An important cause of renal failure after bariatric surgery
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Obesity is a major public health issue all over the world. Bariatric surgery is increasingly becoming popular as a surgical treatment for morbid obesity. Nephrologists need to be aware of possible renal complications after bariatric surgery. We report a 54-year-old male patient who presented with progressive worsening of renal function following a duodenal switch procedure for morbid obesity, and he was found to have oxalate nephropathy on renal biopsy.
Keywords
Bariatric surgery
diabetes
obesity
oxalate nephropathy
Introduction
Oxalate nephropathy has many etiologies and remains a rare cause of renal failure. It is characterized by tubular crystalline deposits of calcium oxalate leading to acute and chronic tubular injury, interstitial fibrosis, and progressive renal insufficiency.
Case Report
A 54-year-old male was seen in our nephrology clinic for progressive increase in his serum creatinine from 1.38 to 3.66 mg/dl over 9 months. There was no history of fever, diarrhea, steatorrhea, urinary tract symptoms, renal stones, or use of nephrotoxic agents. His past medical history included hypertension for 15 years, type 2 diabetes for 11 years, obstructive sleep apnea, depression, and treated cellulitis. He had undergone a duodenal switch procedure for morbid obesity (body mass index >50 kg/m2) 20 months earlier. Subsequently, he had lost more than 100 kg of body weight, and there was significant improvement in the control of blood pressure, sleep apnea, and blood sugar. His serum creatinine was 1.38 mg/dl and urinalysis was normal at the time of surgery.
His clinical examination was unremarkable. Laboratory investigations showed hemoglobin of 9.9 g/dl, white cell count of 6.9 × 103/μl, blood urea nitrogen of 51.82 mg/dl, and serum creatinine of 3.66 mg/dl. Serum electrolytes, calcium, phosphate, and bicarbonate were within normal limits. Urinalysis showed 5-10 white cells/hpf and 1-2 white cell casts without any proteinuria or hematuria. Ultrasound of kidneys showed normal sized kidneys with a few small bilateral calculi without any evidence of obstruction. Doppler of renal arteries was normal.
In view of progressive unexplained increase in creatinine, a kidney biopsy was done. The kidney biopsy [Figures 1 and 2] demonstrated 13 glomeruli of which six were globally sclerosed. The remaining glomeruli were histologically normal. The tubular interstitial compartment showed foci of tubular damage with deposition of translucent crystals of different shapes which are predominantly intraluminal with few intracellular and focal interstitial distributions. Under polarized light, the deposits appeared strongly birefringent forming fan-like, sheaf-like, or irregular shapes consistent with calcium oxalate crystals. There was an associated mild interstitial inflammation with moderate interstitial fibrosis and tubular atrophy. Blood vessels exhibited moderate arteriosclerosis and arteriolar hyalinosis. The immunofluorescence was negative and electron microscopy did not show any electron dense deposits but did reveal tubular damage with intratubular crystal deposition. His 24-h urinary oxalate level was found to be significantly elevated at 98.7 mg/day (normal range upto 40 mg/day). Urinary calcium and citrate were undetectable.
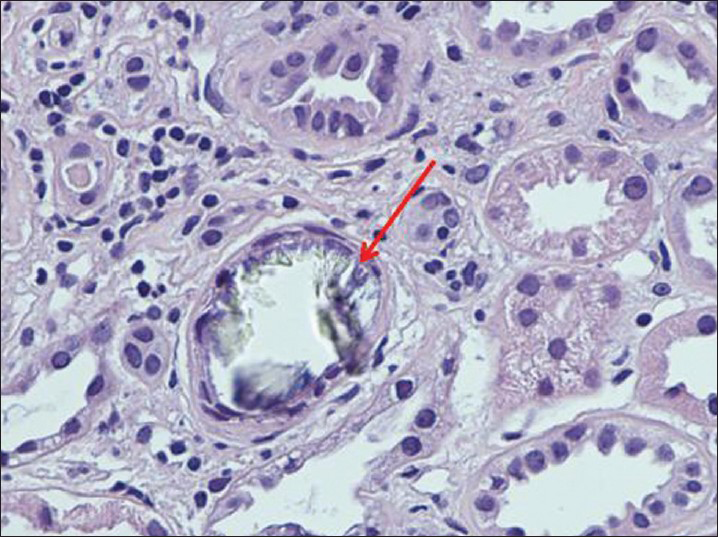
- Large intraluminal translucent crystals of calcium oxalate, tubular epithelial degeneration (foamy cytoplasm, pyknosis, karyorrhexis, indistinct cell borders, dilated lumina), lymphocytic infiltration in the interstitium (H and E, ×400)

- Large intraluminal translucent crystals of calcium oxalate, tubular epithelial degeneration, multinucleated foreign body giant cell reaction (H and E, ×400)
A diagnosis of oxalate nephropathy secondary to enteric hyperoxaluria post-duodenal switch operation was made and he was started on low-oxalate, low-fat diet, with high-fluid intake (>3 liters per day). In addition, he was prescribed calcium citrate 1000 mg tid with meals as an oxalate binder. He was also started on cholestyramine 4 g twice daily and repeat urine oxalate improved markedly to 63 mg/day and his serum creatinine stabilized.
Discussion
We report a case of oxalate nephropathy occurring in the second-year post-duodenal switch surgery for morbid obesity. Estimates from the National Center for Health Statistics show that the prevalence of obesity in the USA exceeds 30% in most age groups across both genders.[1] Presently, gastric banding, Roux-en-Y gastric bypass surgery (RYGB), and duodenal switch operation are the commonly practiced bariatric surgeries.
There is evidence for significant benefit in terms of improvement in hypertension control diabetes, dyslipidemia, urinary albumin excretion, markers of atherosclerosis, and progression of chronic kidney disease (CKD) after weight reduction surgeries.[234] There is also evidence for reduction in mortality and morbidity after bariatric surgery.[5]
With increasing numbers of RYGB and duodenal switch operations being performed, the complication of oxalate nephropathy is coming to light. Oxalate nephropathy is progressive in nature and has a poor prognosis.[67]
With RYGB or duodenal switch surgery, the patient has an increased absorption of oxalates from colon leading to enteric hyperoxaluria. The increase in oxalate absorption and subsequent excretion in this setting is related to fat malabsorption leading to unavailability of free calcium for binding oxalate in gut, and unabsorbed bile salt-induced increased colonic permeability to small molecules, such as oxalate. In 2005, Nelson et al. first described the presence of hyperoxaluria in patients after a standard RYGB.[8] Park et al. measured 24-h urinary collections in 45 patients after bariatric surgery over 2 years[6] and reported that bariatric surgery was almost uniformly associated with increased urinary oxalate and calcium oxalate super saturation. Other important potential factors contributing to nephrolithiasis in this population were decreased urinary volume and decreased urinary citrate.[6] In a large group of 4,639 RYGB patients, the incidence of nephrolithiasis was 7.7% compared with an incidence of 4.6% in an equally large control group of obese patients who had not undergone an operation.[7] Until now, only a few case reports of oxalate nephropathy have been reported after bariatric surgery. In a Mayo clinic series of 60 cases of post-RYGB, only two had oxalate nephropathy and both reached end-stage renal disease (ESRD).[9] Nasr et al. have reported the largest series of oxalate nephropathy with 11 cases diagnosed post-RYGB of which eight reached ESRD.[10] The diagnosis of oxalate nephropathy should be suspected in any patient who presents with sub-acute or chronic renal failure following bariatric surgery. A 24-h urine collection for oxalate excretion to look for hyperoxaluria and a kidney biopsy is needed for confirming the diagnosis of oxalate nephropathy.[10] It is not uncommon to find other renal lesions such as hypertensive glomerulosclerosis, diabetic nephropathy, or obesity-associated focal segmental glomerulosclerosis concomitantly due to the co-morbidities of these patients.[10]
Treatment of oxalate nephropathy post-bariatric surgery targets reduction of enteric hyperoxaluria. A low-fat diet is recommended to reduce the binding of calcium by free fatty acids. Low oxalate and high-fluid intake are also recommended. The use of calcium salts to bind oxalate is the mainstay of therapy, though there is little published on the efficacy of this approach.[11] Calcium citrate may be preferable to calcium carbonate as there is almost universally concomitant hypocitraturia. But the risk of increased aluminum absorption and worsening of bone disease should be kept in mind, especially if the patient has advanced CKD. Cholestyramine may also be helpful as the presence of bile salts in the colon may increase colonic permeability to organic acids such as oxalate.[11]
Conclusion
Though bariatric surgeries have significant benefits in terms of mortality and morbidity, it is important that the clinician be aware of post-procedure risk of developing secondary enteric hyperoxaluria, nephrolithiasis, and renal failure due to oxalate nephropathy.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235-41.
- [Google Scholar]
- Urinary albumin excretion, HMW adiponectin, and insulin sensitivity in type 2 diabetic patients undergoing bariatric surgery. Obes Surg. 2010;20:308-15.
- [Google Scholar]
- Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index <35 kg/m2. Surg Obes Relat Dis. 2010;6:332-8.
- [Google Scholar]
- Bariatric surgery and progression of chronic kidney disease. Surg Obes Relat Dis. 2009;5:662-5.
- [Google Scholar]
- Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-52.
- [Google Scholar]
- A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol. 2009;182:2334-9.
- [Google Scholar]
- Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: Potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481-5.
- [Google Scholar]
- Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100-7.
- [Google Scholar]
- Oxalate nephropathy complicating Roux-en-Y Gastric Bypass: An underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3:1676-83.
- [Google Scholar]







