Translate this page into:
Syndrome of rapid onset end stage renal disease in incident Mayo Clinic chronic hemodialysis patient
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Despite decades of research, a full understanding of chronic kidney disease (CKD)-end stage renal disease (ESRD) progression remains elusive. The common consensus is a predictable, linear, progressive and time-dependent decline of CKD to ESRD. Acute kidney injury (AKI) on CKD is usually assumed to be transient, with recovery as the expected outcome. AKI-ESRD association in current nephrology literature is blamed on the so-called “residual confounding.” We had previously described a relationship between AKI events and rapid onset yet irreversible ESRD happening in a continuum in a high-risk CKD cohort. However, the contribution of the syndrome of rapid onset-ESRD (SORO-ESRD) to incident United States ESRD population remained conjectural. In this retrospective analysis, we analyzed serum creatinine trajectories of the last 100 consecutive ESRD patients in 4 Mayo Clinic chronic hemodialysis units to determine the incidence of SORO-ESRD. Excluding 9 patients, 31 (34%) patients, including two renal transplant recipients, had SORO-ESRD: 18 males and 13 females age 72 (range 50-92) years. Precipitating AKI followed pneumonia (8), acutely decompensated heart failure (7), pyelonephritis (4), post-operative (5), sepsis (3), contrast-induced nephropathy (2), and others (2). Time to dialysis was shortest following surgical procedures. Concurrent renin angiotensin aldosterone system blockade was higher with SORO-ESRD - 23% versus 5%, P = 0.0113. In conclusion, SORO-ESRD is not uncommon among the incident general US ESRD population. The implications for ESRD care planning, AV-fistula-first programs, general CKD care and any associations with renal ageing/senescence warrant further study.
Keywords
Acute kidney injury
chronic kidney disease
end stage renal disease
renal replacement therapy
Introduction
Despite several decades of research efforts devoted to studying the patterns of chronic kidney disease-end stage renal disease (CKD-ESRD) progression as well as the impact of acute kidney injury (AKI) on this continuum of CKD-ESRD evolution, a full understanding of the process (es) of CKD-ESRD progression remains elusive.[1] The common consensus of a predictable, linear, progressive, relentless and time-dependent decline in renal function, with predictably increasing serum creatinine values over time leading inexorably to ESRD is widely accepted.[234567] AKI on CKD is a well-recognized phenomenon, but again this is usually assumed to be transient with usual expected recovery of renal function or at the worst, some loss of residual renal function.[8910] The association between AKI and ESRD is unclear, and often considered as “residual confounding”.[8910]
We have observed and documented acute yet irreversible renal failure in several CKD patients leading to ESRD.[211] Subsequently, we prospectively investigated this syndrome of acutely rapid onset yet irreversible ESRD further in a 100-patient high-risk CKD cohort, the syndrome of rapid onset ESRD (SORO-ESRD).[12] This accelerated progression to ESRD from a-priori stable CKD was precipitated by AKI resulting from medical and surgical events that had then quickly and directly led to established irreversible ESRD without any renal recovery.[21112]
Of the 15 patients with SORO-ESRD first described in 2010, mean age was 68 years, 9 of the 15 (60%) patients were aged 65 years and older and 6 of the 15 (40%) patients were aged 80 years or older.[21112] These observations suggested that this syndrome was more common in the older adult CKD patient and that such acute yet irreversible ESRD may be related to the changes that occur concurrently in the ageing kidney, otherwise described as renal senescence.[1314]
Since this 100 patient cohort was a high-risk CKD cohort because they were recruited into an angiotensin inhibition withdrawal study after demonstrating worsening renal failure while on concurrent angiotensin inhibition, the question had remained unanswered as to what extent this SORO-ESRD phenomenon pertains to the general incident United States (US) ESRD population.[21112]
From the foregoing therefore, in June 2011, we investigated the serum creatinine trajectories in consecutive 100 adult ESRD patients undergoing out-patient in-center maintenance hemodialysis in a Mayo Clinic Dialysis practice to evaluate the incidence of SORO-ESRD in a general US ESRD population.
Materials and Methods
This is a retrospective analysis of individual patient-level serum creatinine trajectories of the last consecutive 100 adult (18 years and older) ESRD patients undergoing out-patient in-center maintenance hemodialysis in four Northwestern Wisconsin Mayo Clinic Hemodialysis Units.
We analyzed, in June 2011, the serum creatinine trajectories of the last 100 consecutive adult ESRD patients on RRT for ≥90 days in four Northwestern Wisconsin Mayo Clinic Hemodialysis Units. The ESRD patients were on maintenance out-patient in-center hemodialysis in four Mayo Clinic hemodialysis units located in the following cities - Eau Claire, Wisconsin,[2] Menomonie, Wisconsin[1] and Barron, Wisconsin.[1] They were seen and managed in these four Mayo Clinic Hemodialysis units between January 2010 and February 2011.
We have defined SORO-ESRD as the unpredictable, unanticipated and accelerated progression from a-priori stable CKD to irreversible ESRD, all occurring in one continuum of time without renal recovery in between, requiring permanent RRT, following a new episode of AKI, caused by antecedent new medical/surgical events, with the need for RRT usually occurring within 6-12 weeks of the AKI episode.[21112] Our working definition of SORO-ESRD is any patient with an estimated glomerular filtration rate (eGFR) of ≥30 ml/min/1.73 m2, on or before the 90th day preceding initiation of first RRT for renal failure and who thereafter had remained permanently on RRT for over 90 days and beyond without renal recovery, indefinitely.[21112]
Results
Due to incomplete serum creatinine data, 9 patients were excluded from the analysis.[289] The remaining 91 ESRD patients included 57 males and 34 females, age range 39-93 years.
Thirty-one of the 91 (34%) ESRD patients satisfied the diagnosis of SORO-ESRD [Figure 1]. These 31 SORO-ESRD patients included 18 males and 13 females, mean age 72 (50-92) years. This SORO-ESRD group of 31 patients also included two renal transplant recipients (RTRs) [Figure 2]. For all 31 SORO-ESRD patients, the abrupt unanticipated and irreversible SORO-ESRD followed AKI event or events resulting from the following causes-pneumonia,[8] acutely decompensated heart failure,[7] pyelonephritis,[4] post-operative states,[5] general sepsis,[3] contrast-induced nephropathy[2] and others.[2] Furthermore, the time interval between the precipitating antecedent AKI event and the resulting need for RRT was relatively shorter following surgical AKI, when compared with medical causes of AKI - usually within days to 1 week, following cardiac surgery [Figure 1 – Bottom]. The 31 SORO-ESRD patients were older compared with the remaining 60 ESRD patients who were characterized by the more traditionally accepted pattern of a slow progressive time-dependent loss of eGFR over time - 71 ± 12 (49-91) years versus 69 ± 13, (38-93) P NS. Incidentally, we observed that 7 of 31 (23%) SORO-ESRD patients were concurrently on renin angiotensin aldosterone system (RAAS) blockade at the time of the diagnosis of AKI versus 3 of 60 (5%) without SORO-ESRD, t (89) =2.587, P = 0.0113.
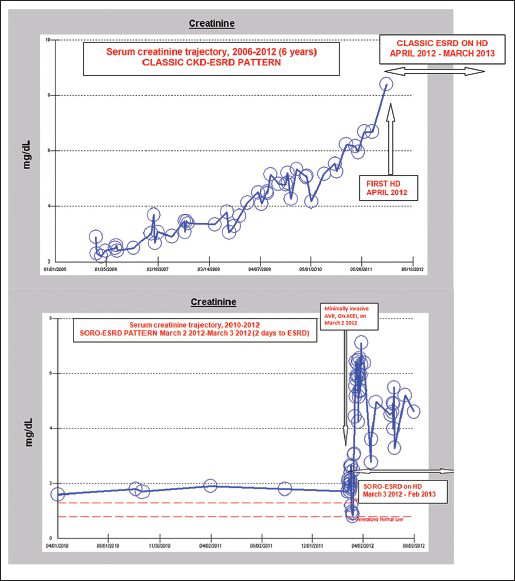
- Composite figure showing the classic chronic kidney disease-end stage renal disease (ESRD) progression pattern of serum creatinine trajectory (top frame) versus the serum creatinine trajectory pattern in another patient with syndrome of rapid onset-ESRD (bottom frame)
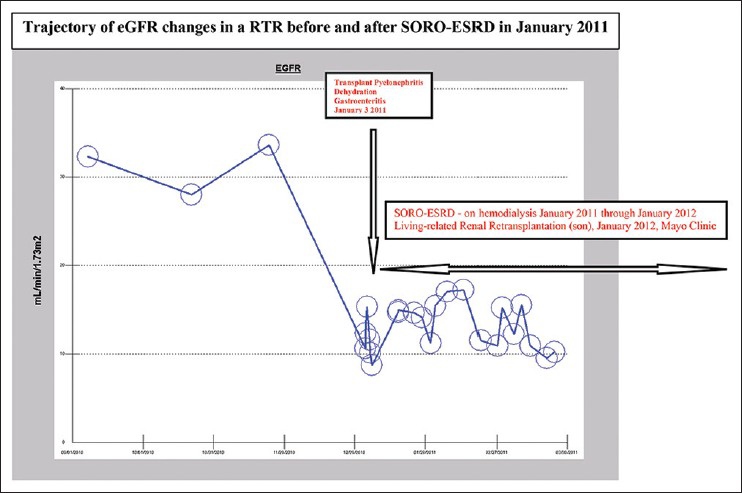
- Trajectory of epidermal growth factor receptor changes in a renal transplant recipient who developed syndrome of rapid onset-end stage renal disease in January 2011 and who subsequently had a second living-related kidney re-transplantation in January 2012 from the son
Retrospective analysis demonstrated that in all the patients where infections triggered AKI, including pneumonia in 8, pyelonephritis in 4, and generalized sepsis in 2, they all responded to appropriate systemic antimicrobial therapy that was initially broad spectrum and was subsequently adjudicated by culture sensitivity results where applicable. There was a deliberate avoidance of potential nephrotoxic antimicrobials especially the aminoglycosides. Post-AKI, in subsequent follow-up, blood pressure control and blood sugar control among the diabetic patients were generally adequate. In patients on concurrent angiotensin inhibition at presentation, this was promptly discontinued, and where applicable, antihypertensive substitution with calcium channel blockers and vasodilators was the practice as we have described previously.[21112]
Discussion
We conclude that SORO-ESRD is not uncommon among the incident US ESRD population as characterized in this Mayo Clinic Northwestern Wisconsin out-patient in-center chronic hemodialysis population.[21112] This retrospective analysis of the individual patient-level serum creatinine trajectories of the last 100 incident adult ESRD patients from four Northwestern Wisconsin Mayo Clinic Hemodialysis Units demonstrated an incidence rate of SORO-ESRD of 34%, about a third of the ESRD population. Our recent review of the AKI literature, 1975-2010, had unearthed 16 individual AKI reports that described patients with features consistent with our working diagnosis of SORO-ESRD.[215] Emphatically, an accompanying editorial to one of these 16 studies, published in the Quarterly Journal Of Medicine in 1996, had referred to similar observations of ESRD rapidly following AKI in patients seen at The General Infirmary at Leeds, United Kingdom as “acute irreversible renal failure”.[16] We have concluded that this indeed was a very apt description of the syndrome after which we had coined the new name, the syndrome of rapid onset end stage renal disease, or SORO-ESRD, in 2010.[2111215]
We suspect that irreversible terminal acute tubular necrosis was the most likely cause of non-recovery in most of our 31 SORO-ESRD patients. This hypothesis is based on clinical observations as related to paucity of urinalysis findings as well as renal sonographic imaging, where available. Renal biopsy was carried out in only one patient, a RTR and the renal pathology demonstrated acute tubular necrosis, changes of chronic glomerulopathy, but without rejection.
It is important to acknowledge here that in all 31 patients who developed SORO-ESRD in this study, the kidney function prior to the AKI insult that precipitated the acute yet irreversible ESRD was otherwise stable. The examination of individual patient-level serum creatinine trajectories of the 31 SORO-ESRD patients demonstrated that serum creatinine values were indeed stable and that eGFR was not declining, prior to the onset of AKI and subsequent development of SORO-ESRD. Such data from four of the 31 patients are shown in the composite figure and it is evident that up until prior to the AKI event that precipitated SORO-ESRD, serum creatinine and therefore eGFR was stable and not changing [Figure 3]. These findings are consistent with our working diagnosis of SORO-ESRD as rapid unanticipated irreversible ESRD following rapidly on AKI in otherwise a-priori stable CKD patients.[21112]

- Composite figure showing stable patterns of pre-acute kidney injury serum creatinine trajectories in 4 of the 31 syndrome of rapid onset end stage renal disease (SORO-ESRD) patients, a pattern that is applicable to all 31 SORO-ESRD patients
Most importantly, in the more recent nephrology literature, 2011-2012, we have further identified three corroborating new reports that have further substantiated our recent reports of SORO-ESRD.[171819] These three new reports have each further demonstrated that a significant proportion of the incident adult ESRD population in both the US and Canada, respectively, satisfy the diagnostic criteria for our newly described SORO-ESRD.[171819]
In a 2011 report, Lee et al., studied all consecutive patients initiated on maintenance hemodialysis or peritoneal dialysis over several years at two dialysis units.[17] According to the investigators, rapid decline in kidney function to ESRD was considered to have occurred if a patient was documented to have estimated GFR >30 ml/min/1.73 m2 within 3 months prior to the initiation of chronic dialysis.[17] Incidentally, we must observe here that their definition very closely mirrored our standard definition of SORO-ESRD.[2111217] Lee et al., revealed that 8 of 105 incident chronic dialysis patients in one dialysis unit (7.6%; 95% confidence interval [CI] 3.4%-14.5%) and 9 of 71 incident patients at another dialysis unit (12.7%, 95% CI 6.0%-22.7%), suffered rapid decline in kidney function that was the immediate precipitant for the need for permanent renal RRT[17] These observations translate to a SORO-ESRD incident rate in this Northern California hemodialysis unit of 15 of 176 or 9%.[17] It is noteworthy to observe that all these patients with rapid irreversible ESRD or SORO-ESRD were started on hemodialysis for RRT, and that all 15 had relied on hemodialysis catheters for initial vascular access.[17] The authors of this report also observed that the patient-level data documentation submitted to the United States Renal Data System (USRDS) did not fully reflect the health status of these patients during their “pre-ESRD” period.[17] As we have continued to argue, the absence of patient-level serum creatinine trajectories in the USRDS database would not allow for an analysis of the USRDS database to estimate the nation-wide incidence of this SORO-ESRD in our incident US ESRD population.[21112] This deficiency in the USRDS database had informed the recent paradigm whereby some eminent nephrologists from around the world, including the US, South America, Europe and the United Kingdom, Africa including Nigeria and Asia and the Indian subcontinent, recently met in Vancouver, Canada, in 2011, at the World Congress of Nephrology Annual Meeting and established “The SORO-ESRD World-wide Consortium” to further investigate this newly described syndrome on a world-wide platform.[2]
Similarly, O’Hare et al., analyzed the trajectories of eGFR during the 2-year period before dialysis initiation in 5,606 Veterans Affairs patients who initiated long-term dialysis between 2001 and 2003.[18] In this report, 9.5% of the ESRD patients had accelerated loss of eGFR from levels >60 ml/min/1.73 m2 (mean eGFR slope, 32.3 ± 13.4 ml/min/1.73 m2 per year) and another 3.1% experienced catastrophic loss of eGFR from levels >60 ml/min/1.73 m2 within 6 months or less to reach irreversible ESRD.[18] The authors of this report had concluded that there was substantial heterogeneity in patterns of kidney function loss leading up to the initiation of long-term dialysis, perhaps calling for a more flexible approach toward preparing for ESRD.[18] Our post-hoc analysis of this Seattle report demonstrated a SORO-ESRD incidence rate in this population of 5,606 Veterans Affairs ESRD patients of 12.6%.[18]
In addition, in a 2012 Canadian report, Siddiqui et al., had described the increasing use of acute dialysis after cardiac surgery in the period 1995 to 2009 and had demonstrated that the incidence of acute dialysis had increased steadily from 0.2% in 1995 (95% CI 0.15-0.2) to 0.6% in 2009 (95% CI 0.6-0.7).[19] Moreover, this study had validated that among the 1294 patients who received acute dialysis and survived beyond 90 days, 352 patients subsequently required chronic dialysis (27.2%, 95% CI 24.8-29.7).[19] We have deduced, from a post-hoc analysis of this study that the majority of the cohort of 352 patients requiring chronic dialysis following post-operative AKI had developed SORO-ESRD, giving a SORO-ESRD incidence rate of 16%.[1920]
We conclude that SORO-ESRD is not uncommon among incident adult ESRD patients on maintenance hemodialysis in the US and in Canada.[211121517181920] SORO-ESRD accounted for 34% of the incident ESRD patients in a Mayo Clinic chronic ESRD population,[21112] 9% of a Northern California incident ESRD population,[17] 12.6% of the Seattle Washington Veterans Affairs incident ESRD population[18] and 16% of the Canadian incident ESRD population,[1920] respectively. The implications of this phenomenon of the SORO-ESRD with regards to ESRD care planning, AV Fistula first programs and overall CKD care in general, are huge and warrant further study.[2111216171819] For example, in the Northern California study, all 15 patients with SORO-ESRD had started hemodialysis using hemodialysis catheters because there was no time for arteriovenous Fistula (AVF) planning in the first instance.[17] If SORO-ESRD is shown to be this prevalent among the general incident US (and world-wide) ESRD population in multi-center studies, major paradigm shifts must be warranted in the way we practice nephrology both here in the US and around the world.[2111217] Maybe, as suggested by Lee et al., the USRDS may begin to request for more “pre-ESRD” patient-level data from submitting Nephrologists.[17]
We must acknowledge here that the impact of AKI in CKD-ESRD progression and pathogenesis remains fertile grounds for ground-breaking research.[221] Furthermore, subgroup analysis of the 31 SORO-ESRD patients versus the 60 who developed classic ESRD revealed that 9 of 31 (29%) SORO-ESRD patients were 80 years and older. This was not any different from the 17 of 60 (28%) patients who developed classic ESRD. The only evident different characteristics observed between these two sub-groups of 80 and over was the higher exposure to angiotensin inhibition in the SORO-ESRD group (4 of 9 [44%] vs. 1 of 17 [6%], 2 tailed Z-test, P = 0.019). Overall, 7 of 31 (23%) SORO-ESRD patients were concurrently on RAAS blockade at the time of the diagnosis of AKI versus 3 of 60 (5%) without SORO-ESRD, t (89) =2.587, P = 0.0113. The plausible contribution of older age and the role of exposure to nephrotoxics, potentially including angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers, to the incidence of SORO-ESRD remain speculative at this point and require further study.[211121314] From this analysis, we submit that there is some plausible role for nephrotoxics including RAAS blockade in the exacerbation of renal outcomes with AKI on CKD.[21112212223]
We would submit that our findings from this study only further strengthen our previous calls for more research efforts into preventative nephrology practices, especially our recently described the concept of renoprevention.[24] Renoprevention is the preemptive withholding of nephrotoxics including RAAS blockers, the aggressive prevention of perioperative hypotension, the avoidance or minimalization of nephrotoxic exposure from iodinated contrast and relevant antibiotics, during critical illness and in the peri-operative period.[15242526] Such a paradigm, it is hoped, will consequently lead to less AKI events and therefore potentially less SORO-ESRD, better patient outcomes and significant dollar savings as we have demonstrated in a recent analysis in the critical care unit of a Northwestern Wisconsin Mayo Clinic Health System hospital.[1525] Such needed paradigm shifts would constitute major rethinking in current nephrology practices, a form of nephrology practice reengineering.[215242526]
Besides, the possible interplay of renal senescence and changes in the renal anatomy with ageing such as senile hypofiltration (glomerulosclerosis, mesangial expansion) and renal vascular changes (renal atherosclerosis, vascular dysautonomy, arteriole subendotelial hyalinosis, aglomerular circulation) and the pathogenesis of this SORO-ESRD in the older (>65 year old) adult CKD patient is of major research interest to our group.[1314] The fact that in our first report of SORO-ESRD in 2010, older age appeared to be a significant pathogenetic factor in the development of the syndrome is again acknowledged.[21112] The finding in this study that the 31 SORO-ESRD patients versus the remaining 60 classic ESRD patients were older, further strengthened this hypothesis of renal ageing and renal senescence being risk factors for this previously unrecognized syndrome. These observations call for more research in these directions and may imply that the older CKD patient may necessarily be treated differently especially with respect to the use of certain potentially nephrotoxic agents including the RAAS blocking agents.[211121314222327] We have posited the question: Does the older CKD adult patient demand to be treated any differently from the younger CKD patient? The answers remain to be determined.
There are several plausible explanations that could justify why SORO-ESRD is commoner among our older or aged patients.[211121314] On one hand, there are several structural and physiological changes in the aged kidney, such as senile renal blood flow reduction, artery atherosclerosis (renal arterial stenosis) and vascular dysautonomia (impaired vascular autoregulation), which can worsen renal parenchymal ischemia when the aged patient is exposed to hypovolemia (dehydration) or other renal insults (sepsis, cardiac failure).[1314] Besides, GFR reduction secondary to senescence (senile glomerulosclerosis), could represent a risk of installing SORO-ESRD in states of hemodynamic instability, particularly in the oldest old.[28] Moreover, aging tubular cells may be more vulnerable to ischemic injury because cellular antioxidant defenses decline with age and oxidant injury may be a critical determinant of ischemic acute renal failure.[131428] What's more, it was also documented that an increased propensity to vasoconstriction may enhance susceptibility of old kidney to toxic substances.[282930] This senile tubular frailty predisposes the aged kidney to easily develop acute tubular necrosis, as well as to delay its functional recovery, thus predisposing to the prolonged acute tubular necrosis, or even non-recovery that is usually observed during acute renal failure in elderly patients.[282930] Furthermore, renal and hepatic senescence, as well as polypharmacy make old people more susceptible to develop severe acute renal failure when they receive potential nephrotoxic substances such as non-steroidal anti-inflammatory or radio-contrast, which for different reasons are more frequently prescribed in this age group.[283031]
Regarding ACE inhibitors and angiotensin receptor blockers, even though these drugs are widely used for renoprotection in CKD, it must be taken into account that they can induce acute renal failure in those patients who suffer from disorders, prevalent in the elderly, in which maintenance of GFR is highly dependent on an angiotensin II-mediated efferent vasoconstriction.[21112323334] This is true for patients with bilateral renal artery stenosis, renal artery stenosis in a solitary kidney, congestive heart failure and severe renal failure, especially when they are volume depleted.[32] Finally, several causes of rapidly progressive glomerulopathies, which are frequent among the older patient, such as crescentic glomerulonephritis, systemic vasculitis, atheroembolic disease, could be another causal mechanism of SORO-ESRD in this older population.[35]
Lastly, the fact that 2 of the 31 (6%) of the SORO-ESRD patients in our present study were RTRs raises another significant concern – to what extent is renal allograft loss attributable to this SORO-ESRD?.[28936] The full details of the presentations of these two RTRs who developed SORO-ESRD, one of whom was re-transplanted in January 2012, a year after developing SORO-ESRD, with a living related renal allograft from her 32-year old son at Mayo Clinic, Rochester, the first such report of SORO-ESRD among RTRs, has just been published in this journal, the Indian Journal of Nephrology, in 2013.[36] These unanswered questions call for further research and urgent answers.
In closing, we must observe that despite decades of painstaking research into the dynamics, processes and mechanisms of CKD-ESRD propagation and progression, the medical community remains at a loss in understanding the nuances of these translations.[1] The common CKD staging classification represents an assumed and arguably untested paradigm that in our opinion, had certainly increased awareness of CKD, without truly advancing our knowledge in this field of medicine, nor have we been able to make any major break-through achievements in improving CKD outcomes.[37] Tragically, this approach may be counter-productive as it continues to buttress the notion that CKD is a homogenous clinical entity and that all “CKD is equal.”[37] This notion is so much further from the truth.[37] We suggest a complete reappraisal of current nephrology practices and to begin to develop new models of CKD care that correctly recognize the diversity of CKD as representative of a wide spectrum of disease states.[37] Most appropriately, Bansal and Hsu, in a 2008 analysis of the long-term outcomes of patients with CKD had strongly echoed the observation that the disparate ESRD and mortality rates in various CKD populations as reported by various studies in the literature only emphasized the heterogeneity of different CKD populations.[38] Nephrologists must not rely on CKD staging alone to direct management of or risk-stratification of patients with CKD, in general, but must always consider the etiology and rate of progression of kidney disease, patient age and a wide array of cardiovascular disease risk factors.[37] The overarching need to always individualize CKD care cannot be overemphasized as CKD represents a whole wide spectrum of distinctly different clinical disease entities, with each individual patient often subject to a multitude of aggravating factors, some of which often remain unrecognized.[211123738] This is in fact how we practice medicine - one patient and only one patient at a time.[37]
Source of Support: Nil
Conflict of Interest: None declared.
References
- Acute kidney injury: A springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078-94.
- [Google Scholar]
- The syndrome of rapid onset end-stage renal disease – A new mayo clinic dialysis services experience, January 2010-February 2011. In: Di Iorio B, Heidland A, Onuigbo M, Ronco C, eds. Hemodialysis: How, When and Why. Hauppauge NY: NOVA Science Publishers; 2012. p. :443-85.
- [Google Scholar]
- The GISEN Group. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Lancet. 1997;349:1857-63.
- [Google Scholar]
- Outcomes of stage 3-5 chronic kidney disease before end-stage renal disease at a single center in Taiwan. Nephron Clin Pract. 2008;109:c109-18.
- [Google Scholar]
- Risk factors for progression in patients with early-stage chronic kidney disease in the Japanese population. Intern Med. 2008;47:1859-64.
- [Google Scholar]
- Predicting mortality and uptake of renal replacement therapy in patients with stage 4 chronic kidney disease. Nephrol Dial Transplant. 2009;24:1930-7.
- [Google Scholar]
- Long-term functional evolution after an acute kidney injury: A 10-year study. Nephrol Dial Transplant. 2008;23:3859-66.
- [Google Scholar]
- Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179-85.
- [Google Scholar]
- Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223-8.
- [Google Scholar]
- Chronic Kidney Disease and RAAS Blockade: A New View of Renoprotection. London, England: Lambert Academic Publishing GmbH 11 and Co. KG; 2011.
- [Google Scholar]
- Syndrome of rapid-onset end-stage renal disease: A new unrecognized pattern of CKD progression to ESRD. Ren Fail. 2010;32:954-8.
- [Google Scholar]
- Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011;119(Suppl 1):p1-5.
- [Google Scholar]
- Evidence of the syndrome of rapid onset end-stage renal disease (SORO-ESRD) in the acute kidney injury (AKI) literature-Preventable causes of AKI and SORO-ESRD - A call for re-engineering of nephrology practice paradigms. Ren Fail. 2013;35:796-800.
- [Google Scholar]
- End-stage renal disease preceded by rapid declines in kidney function: A case series. BMC Nephrol. 2011;12:5.
- [Google Scholar]
- Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis. 2012;59:513-22.
- [Google Scholar]
- Secular trends in acute dialysis after elective major surgery – 1995 to 2009. CMAJ. 2012;184:1237-45.
- [Google Scholar]
- Increasing trends in acute dialysis after elective surgery, 1995-2009: How often does AKI directly lead to irreversible ESRD all in one fell swoop? Can Med Assoc J Published Online 2012 July 13
- [Google Scholar]
- Survivors of acute renal failure who do not recover renal function. QJM. 1996;89:415-21.
- [Google Scholar]
- Late onset renal failure from angiotensin blockade: A prospective thirty-month mayo health system clinic experience. Med Sci Monit. 2005;11:CR462-9.
- [Google Scholar]
- Late-onset renal failure from angiotensin blockade in 100 CKD patients. Int Urol Nephrol. 2008;40:233-9.
- [Google Scholar]
- Reno-prevention vs. reno-protection: A critical re-appraisal of the evidence-base from the large RAAS blockade trials after ONTARGET – A call for more circumspection. QJM. 2009;102:155-67.
- [Google Scholar]
- Renoprevention: A new concept for reengineering nephrology care – An economic impact and patient outcome analysis of two hypothetical patient management paradigms in the CCU. Ren Fail. 2013;35:23-8.
- [Google Scholar]
- The nephrotoxic “triple whammy” of combining diuretics, ACE inhibitors, and NSAIDs [corrected] BMJ. 2013;346:f678.
- [Google Scholar]
- Can ACE inhibitors and angiotensin receptor blockers be detrimental in CKD patients? Nephron Clin Pract. 2011;118:c407-19.
- [Google Scholar]
- Geriatric nephrology and the ‘nephrogeriatric giants’. Int Urol Nephrol. 2002;34:255-6.
- [Google Scholar]
- Acute renal failure in the elderly: particular characteristics. Int Urol Nephrol. 2006;38:787-93.
- [Google Scholar]
- Renal handling of water and electrolytes in the old and old-old healthy aged. In: Núñez M, Cameron S, Oreopoulos D, eds. The ageing kidney in health and disease. New York: Springer; 2008. p. :141-54.
- [Google Scholar]
- Angiotensin I converting enzyme inhibitors and angiotensin II receptor antagonist. In: De Broe M, Porter G, Bennett W, Deray G, eds. Clinical Nephrotoxins. New York: Springer; 2008. p. :481-94.
- [Google Scholar]
- Angiotensins. In: Fray JC, ed. Handbook of Physiology: The Endocrine System: Endocrine Regulation of Water and Electrolyte Balance. Vol 3. New York, Oxford: Oxford University Press; 2000. p. :104-54.
- [Google Scholar]
- Angiotensin converting enzyme inhibitors. In: DeBrue AN, ed. Angiotensin Converting Enzyme Inhibitors. New York: Nova Biomedical Books Published by Nova Science Publishers; 2009. p. :1-41.
- [Google Scholar]
- The diagnosis of renal diseases in elderly patients. What role is there for biopsy? In: Núñez M, Cameron S, Oreopoulos D, eds. Renal Ageing: Health and Disease. New York, US: Springer; 2008. p. :307-27.
- [Google Scholar]
- Syndrome of rapid-onset end-stage renal disease in two consecutive renal transplant recipients. Indian J Nephrol. 2013;23:222-5.
- [Google Scholar]
- The CKD enigma with misleading statistics and myths about CKD, and conflicting ESRD and death rates in the literature: Results of a 2008 U.S. population-based cross-sectional CKD outcomes analysis. Ren Fail. 2013;35:338-43.
- [Google Scholar]
- Long-term outcomes of patients with chronic kidney disease. Nat Clin Pract Nephrol. 2008;4:532-3.
- [Google Scholar]







