Translate this page into:
Renal cortical necrosis: A rare complication of Plasmodium vivax malaria
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A young female with Plasmodium vivax malaria presented with anemia, hyperbilirubinemia, thrombocytopenia, and advanced renal failure. She remained anuric for more than 3 weeks. Kidney biopsy confirmed the diagnosis of acute cortical necrosis. During follow-up, she became dialysis independent, but remained in stage 4 chronic kidney disease (CKD) at 3 month. P. vivax is supposed to be benign in nature, but can lead to rare and severe complication like renal cortical necrosis and progress to CKD.
Keywords
Acute kidney injury
malaria
Plasmodium vivax
renal cortical necrosis
Introduction
Plasmodium vivax malaria is usually an uncomplicated disease, which runs a benign course. In the last few years, changing trends with severe and life-threatening complications due to P. vivax has been reported from different endemic regions such as India, Indonesia, and Brazil.[12] Complications similar to falciparum malaria such as hypotension, cerebral malaria, multiorgan dysfunction, hypoglycemia, thrombocytopenia, renal impairment, and hepatic dysfunction have been published. These manifestations may be due to the change in the clinical spectrum of disease, increase in resistance, indiscriminate use of anti-malarial drugs, delayed treatment, or missing the primaquine therapy.[34]
Here, we present a case of acute cortical necrosis (ACN) caused by P. vivax monoinfection in a young female from endemic zone of malaria which subsequently progressed to chronic kidney disease (CKD).
Case Report
A 17-year-old female was referred from a primary health center in an endemic zone of malaria. She had a history of fever with chills, rigor, nausea, and headache. She developed reddish discoloration of urine 3-4 days following the onset of fever with progressive decrease in urine output and became anuric. She developed symptoms of fluid overload and was referred to us. There was no history of petechial hemorrhage, rash, loose stool, vomiting, cough, burning micturition, altered sensorium, or seizures.
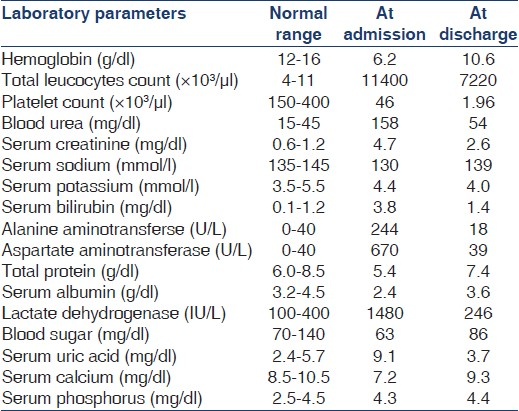
On examination, she was conscious, oriented, dyspneic at rest and had pallor, icterus, and anasarca. She was afebrile. She had pulse rate of 112/min, respiratory rate of 32/min and blood pressure was 170/100 mmHg. Chest examination revealed bilateral crepitations. Cardiovascular examination was normal. Abdominal examination showed mildly enlarged liver with free fluid in the abdomen and had palpable spleen. Her investigations at the time of admission and at discharge mentioned in detail in Table 1. Diagnosis of P. vivax malaria was confirmed by rapid diagnosis test (negative histidine-rich protein 2 of P. falciparum and positive P. vivax specific lactate dehydrogenase) and peripheral blood smear examination. Ultrasonography of abdomen showed normal size kidney with mild liver and spleen enlargement. Arterial blood gas showed hypoxemia with metabolic and respiratory acidosis. Patient was put on mechanical ventilator and received acute peritoneal dialysis. She was managed with WHO regimen of malaria and broad-spectrum intravenous antibiotic therapy. In next 2 days, patient's respiratory parameters improved and she was weaned-off from the mechanical ventilation.
Serological tests for viral hepatitis, dengue, leptospirosis and Widal test were negative. Her urine examination showed 2+ proteinuria, red blood cells (RBCs) and white blood cells per high power field. Coomb's test, C-reactive protein, D-dimer and fibrin degradation product level, antinuclear antibody, antidouble stranded DNA, antineutrophil cytoplasmic antibody (ANCA) cytoplasmic-ANCA and perinuclear-ANCA, complement 3 and 4, antiglomerular basement membrane antibody were normal. Blood, urine and sputum culture were sterile. She had progressive decrease in hemoglobin level, platelet count, and increasing levels of serum urea and creatinine. Diagnosis was kept as severe complicated malaria due to P. vivax with acute kidney injury, pulmonary edema, metabolic acidosis, hemolytic anemia, thrombocytopenia, and hyperbilirubinemia.
She was kept on hemodialysis, and received six units of packed RBCs and five unit of platelet concentrates. Kidney biopsy was done as renal function remained abnormal even after 3 weeks [Figures 1–3] Kidney biopsy had 15 glomeruli which showed coagulative necrosis involving 9 glomeruli. Other glomeruli were unremarkable except mild mesangial hypercellularity. Interstitium showed edema and moderate infiltration by lymphoplasmacytoid cells. Tubules showed RBCs and RBC casts. Immunofluorescence was negative.
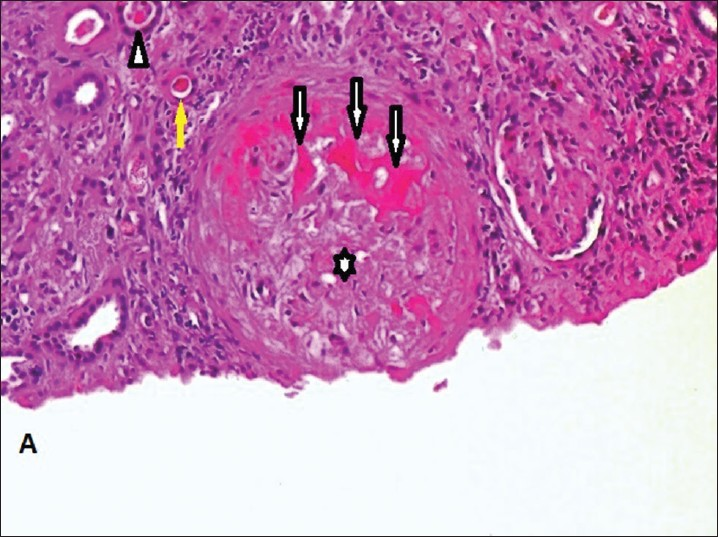
- Renal cortical necrosis. Coagulative necrosis of glomeruli (arrow); mild mesangial hypercellularity (asterix); tubules show presence of red blood cells and red blood cell casts (arrow head) and coagulative necrosis (yellow arrow) (H and E, ×100)

- Presence of brown pigment in glomerular portion (blue arrow); coagulative necrosis of glomeruli (black arrow); red blood cell casts in tubule (yellow arrow); coagulative necrosis in tubules (green arrow); interstitium show edema and infiltration by lymphoplasmacytoid cells (black star) (H and E, ×100)

- Silver stain shows features of coagulative necrosis of tubule and tubular red blood cell casts with interstitial infiltration of mononuclear cells (×100)
During hospital stay her urine output started increasing and she became dialysis independent. She was discharged on day 43 at serum creatinine of 2.6 mg/dl (Modification of Diet in Renal Disease [MDRD] glomerular filtration rate [GFR] of 25.8 ml/min/1.73 m2). She was on follow-up every month, at 3 months after discharge her serum creatinine was 2.4 mg/dl (MDRD GFR of 28.3 ml/min/1.73 m2); in stage 4 CKD.
Discussion
P. falciparum infection causes severe and potentially fatal malaria[56] and might present with severe complications like thrombocytopenia, acute tubular necrosis (ATN), acute respiratory distress syndrome (ARDS), cerebral malaria, hypoglycemia, hypotension, and shock.[6]
P. vivax malaria (benign tertian malaria) is usually uncomplicated and is rarely fatal. In recent times, trends may be changing and there are reports of severe malaria due to P. vivax infection.[47]
Severe malaria results from a combination of parasite-specific factors, such as adhesion and sequestration in the vasculature and the release of bio-active molecules, together with host inflammatory responses. P. vivax preferentially infects young RBCs, parasitemia rarely exceed 2% of circulating RBCs, and high parasite burdens are not a feature of severe disease.[8] However, cytokine production during P. vivax infections is higher than P. falciparum infections of similar parasite biomass.[9]
In their study Andrade et al.,[10] found a strong linear trend between increased levels of C-reactive protein, tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), IFN-γ/interleukin-10 ratio and the disease severity of P. vivax malaria. Price et al.,[11] reported that the plasma concentrations of TNF-α are higher in P. vivax when compared to P. falciparum malaria with similar degree of parasitemia.
Acute renal failure caused by P. vivax malaria has been reported. ATN due to renal ischemia is the predominant mechanism.[8] Several hypotheses have been described including mechanical obstruction caused by cytoadherence and sequestration of infected erythrocytes, immune-mediated glomerular pathology, and release of cytokines.[11]
P. vivax can cause both sequestration-related complications such as cerebral malaria, renal dysfunction, hepatic dysfunction, and ARDS and non sequestration-related complications such as anemia and thrombocytopenia.[4]
Here, we reported a case of ACN, an unusual complication of P. vivax monoinfection. This case illustrates that this parasite, which was supposed to be benign, can lead to serious complication like CKD. One previous report from Rajasthan[12] may explain the possible geological contribution of this region. This region is the part of Thar Desert with hot and dry climate, which may exaggerate the clinical spectrum of the infectious diseases.
A careful consideration of ACN is required in patients who have prolonged oligo-anuria. P. vivax malaria can lead to CKD if such an adverse and rare complication develops. An integrated multidisciplinary approach is needed to study the changing spectrum of P. vivax infection. Field studies, laboratory, molecular, and genomic methods can provide the way for better understanding of disease pathogenesis, prevention, control and treatment of malaria in endemic countries. Epidemiologic studies, clinical description, and comparison of P. vivax with P. falciparum are needed to understand the dynamics and its interaction with the immune system.
Conclusion
Our case report highlights the fact that P. vivax monoinfection malaria can have wide range of presentation similar to P. falciparum and lead to serious, potentially life-threatening complications. It can lead to AKI due to ACN and progress to CKD.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: A prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128.
- [Google Scholar]
- Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis. 2010;16:1611-4.
- [Google Scholar]
- Plasmodium vivax malaria-associated acute kidney injury, India, 2010-2011. Emerg Infect Dis. 2012;18:842-5.
- [Google Scholar]
- Severe Plasmodium vivax malaria: A report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194-8.
- [Google Scholar]
- Malaria. In: Manson's Tropical Diseases (22nd ed). London: Elsevier Saunders; 2011. p. :1201-82. Ch. 73
- [Google Scholar]
- Clinical manifestations of complicated malaria-an overview. J Indian Acad Clin Med. 2003;4:323-31.
- [Google Scholar]
- Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J. 2010;9:13.
- [Google Scholar]
- Renal cortical necrosis and acute kidney injury associated with Plasmodium vivax: A neglected human malaria parasite. Parasitol Res. 2012;111:2213-6.
- [Google Scholar]







