Translate this page into:
The safety and efficacy of high dose ferric carboxymaltose in patients with chronic kidney disease: A single center study
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ferric carboxymaltose (FCM) is a parenteral, dextran-free iron formulation designed to overcome the limitations of existing intravenous (IV) iron preparations. We investigated the safety and efficacy of high dose administration of FCM in our anemic chronic kidney disease (CKD) patients. It was a prospective observational study from June 2011 to August 2013. FCM was administered as IV infusion 1000 mg in 250 ml of normal saline over 15-30 min. Efficacy was evaluated by comparing the Hb and/or serum iron status at the first follow-up visit after the infusion with that at the baseline. A total of 500 infusions were administered to 450 patients. All patients had a successful administration of the FCM. None of the patients had any serious drug-related AE. AE of mild to moderate severity observed or reported after the infusion were: accelerated hypertension (0.2%), feeling abnormal (0.6%), headache and bodyaches (0.6% each), and infusion site reaction (0.8%). 261 patients had a follow up Hb, which showed an increase of 1.7 ± 1.5 g/dl after a period of 11 ± 7.2 weeks (P = 0.001); 188 (72%) patients had a rise in Hb of ≥1 g/dl. The increase in Hb was observed uniformly across all stages of CKD. Proportions of patients with an Hb of above 10 and 11 g/dl increased from 30.2% to 62.8% and 16.1% to 37.9%, respectively (P = 0.001). Iron status evaluation done in 44 patients after a follow up period of 15.1 ± 11.5 weeks showed increases in Hb of 1.6 ± 2.2 g/dl (P = 0.001), transferrin saturation of 9.1 ± 16.9% (P = 0.001), and ferritin of 406 ± 449 ng/ml (P = 0.001). We conclude high dose administration of FCM is safe and well-tolerated. It was effective in the treatment of iron deficiency in nondialysis and peritoneal dialysis CKD patients.
Keywords
Anemia
chronic kidney disease
dialysis
efficacy
ferric carboxymaltose
high dose
intravenous iron
iron deficiency anemia
safety
Introduction
Anemia management is an important component of reducing morbidity and improving the quality of life for chronic kidney disease (CKD) patients. Iron deficiency is common in nondialysis CKD (ND-CKD) patients and is most pronounced in hemodialysis (HD-CKD) patients.[12] Iron replacement strategies include the use of oral or intravenous (IV) iron formulations. In ND-CKD patients, Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Anemia in CKD (2012) recommends either oral or IV iron for treatment of iron deficiency.[3] However, oral iron therapy can be compromised by gastrointestinal (GI) side effects in >35% of patients[4] that can result in poor patient compliance and suboptimal iron absorption. IV iron therapy is recommended for HD-CKD patients due to frequent blood losses associated with dialysis machine use and inability to achieve optimum outcomes with oral iron.[35]
Currently available IV iron agents vary in indication, dosing regimens and safety profiles. The maximum dose that can be given in a single visit is limited by the stability of the various iron–carbohydrate moieties. Most CKD patients with iron parameters below KDIGO goals will require a minimum dose of 1000 mg of elemental iron for iron store replenishment and to raise their hemoglobin (Hb).[3] This would necessitate numerous doses of sodium ferric gluconate[6] and iron sucrose depending on indication.[7] There is an unmet need for an IV iron agent that is safely and rapidly administered in larger single doses resulting in less frequent clinic visits and fewer venipunctures. The use of IV iron can be limited by anaphylactic reactions due to dextran-containing iron formulations.
Ferric carboxymaltose (FCM) is a stable, nondextran-containing iron formulation that is believed to allow iron uptake by the reticuloendothelial system with minimal release of free iron.[8] FCM was developed for rapid IV administration in high doses with controlled delivery of iron into target tissues. The pH of the FCM complex is nearly neutral (5.0-7.0) with a physiologic osmolarity.[9] There is no cross-reactivity with dextran and the iron–carbohydrate complex is more stable than that of ferric gluconate or iron sucrose.[1011] Unlike earlier forms of IV iron, FCM can be given in a dose providing up to 1000 mg of iron administered as a rapid infusion over 15 min without the need for a test dose.[8]
Clinical trials of FCM versus comparators conducted with over 10,000 patients with iron deficiency anemia (IDA) associated with a variety of medical conditions have shown improvements in Hb levels and replenishment of depleted iron stores following FCM administration.[121314151617181920] In patients with ND-CKD, FCM was more effective and better tolerated than oral iron for treatment of iron deficiency.[1718] The safety and efficacy of IV FCM has been reported in HD-CKD.[1920] Though, FCM has been approved in Europe since 2007 for the treatment of iron deficiency when oral iron preparations are ineffective or cannot be used, it got approval in India in February 2011. The objective of the present study was to evaluate the safety and efficacy of high dose FCM administration in anemia of CKD.
Materials and Methods
Study design and treatment
The primary objective of this trial was to assess the safety of IV iron supplementation as FCM in anemic CKD patients. The secondary objective was to evaluate the efficacy of FCM in correcting iron deficiency and improving the Hb levels in this patient population on stable doses of erythropoiesis-stimulating agents (ESA). It was a prospective observational study carried out at a government owned tertiary care hospital in North India over a period of 2 years and 3 months (June 2011 to August 2013). It included anemic CKD patients ≥18 years of age with an estimated glomerular filtration rate (GFR) <60 ml/min/1.73 m2. Patients were excluded if they had received parenteral iron therapy or blood transfusion within the last 3 months, were pregnant, or had a history of recent malignancy, infection, GI bleed or major surgery. Serum Hb, transferrin saturation (TSAT), ferritin and creatinine were determined by means of standard laboratory methods. The subjects were stratified by severity of CKD based on GFR, and baseline Hb. Laboratory tests were obtained at baseline and at the first follow up visit.
FCM [Encicarb®; Emcure Pharmaceutical Ltd. Pune, India] was administered as IV infusion 1000 mg in 250 ml of normal saline over 15-30 min. The interval between the repeat FCM administrations was 6 months. In all the patients, blood pressures (BP) were monitored immediately before and after the infusion. In patients with uncontrolled BP before the infusion, the infusion was deferred till the control of BP. Any adverse events (AE) observed during the infusion and observed or reported to have occurred within 24 h of the infusion were recorded.
If the patient was already on an oral iron preparation, the preparation was stopped. The patients, who were already getting ESA, continued and maintained a stable dose during the study. In other patients with Hb <10 g/dl, an ESA was started after FCM administration. Patient with Hb <7 g/dl were treated with erythropoietin (EPO) 10,000 IU weekly and for those with Hb ≥7 g/dl were treated with a EPO dose of 4000-8000 IU weekly. The starting ESA dose was maintained for the duration of the study unless safety concerns dictated a change.
Assessment
The safety population included all subjects who received the FCM during the study period. Safety and tolerability were assessed by the incidence, severity and relation to study medication of AEs, recorded after administration of the FCM. Efficacy analyses were based on a modified intent-to-treat (mITT) population that consisted of all subjects who had results of Hb and/or serum iron status at the first follow up visit following the first FCM infusion. Efficacy was assessed by correction of patients’ Hb levels and iron stores. The Hb and/or TSAT and ferritin levels, at the first follow up visit were compared with those done at the baseline. Treatment responders were defined as patients who exhibited an increase of ≥1.0 g/dl in Hb from baseline on follow up. The study was approved by the institutional Ethics Committee and all the participants provided written informed consent.
Statistical analysis
Descriptive statistics including means, standard deviation and percentages were used to describe the demographic and clinical data. The paired samples t-test with 95% confidence intervals (CIs) was done to compare the mean changes in Hb and iron indices before and after the iron administration. Treatment differences for proportions were assessed using Chi-square test with Fisher's exact test. A difference was considered significant when the P < 0.05. All statistics were carried out using Statistical Package for the Social Sciences (SPSS), version 16 (SPSS, Chicago, IL, USA).
Results
Demographics and clinical characteristics (n = 450)
Of 725 patients screened, 450 were included in the study. The demographics and baseline characteristics of the study patients are shown in Table 1. Two hundred and fifty-three (56.2%) were male and 197 (43.8%) were female. The mean age of the study patients was 57.4 ± 12.9 (range of 18-90) years. The etiology of CKD was diabetic nephropathy, 145 (32.2%), hypertension, 119 (26.4%), chronic tubulointerstitial nephritis, 106 (23.6%), chronic glomerulonephritis, 67 (14.9) and autosomal dominant polycystic kidney disease, 13 (2.9%). Majority (94%) of the patients were ND-CKD: 154 (34.2%) stage 3, 108 (24%) stage 4 and 161 (35.8%) stage 5 of CKD. Twenty seven (6%) patients belonged to stage 5D undergoing maintenance dialysis: 21 (4.7%) peritoneal dialysis-CKD (PD-CKD) and 6 (1.3%) HD-CKD. One hundred and seven (23.8%) patients had previous iron therapy and 70 (15.6%) were already getting ESA therapy. One patient had history of intolerance to IV iron sucrose.
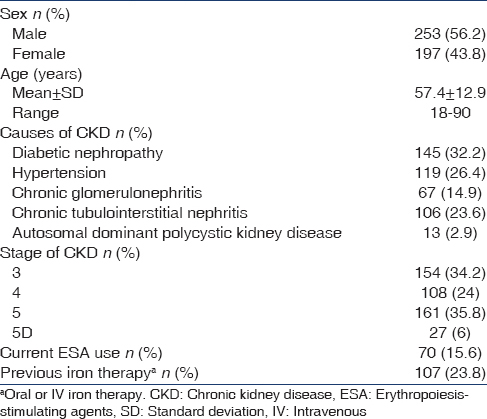
Table 2 shows the baseline investigations of the study patients. The patients had a baseline Hb of 9.6 ± 2.2 g/dl and creatinine of 4.5 ± 3 mg/dl. The iron studies were done in a total of 373 (82.9%) study patients. One hundred and twenty-six (33.8%) of them had raised C-reactive protein (CRP) levels. The mean value of TSAT was 24.8 ± 21.3% and ferritin levels were 265 ± 225 ng/ml.

Safety and tolerability of high dose ferric carboxymaltose administration (n = 500)
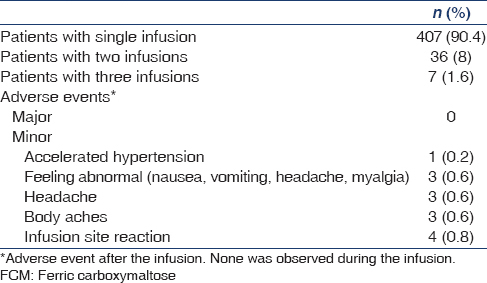
During the study period, 450 patients were treated with a total of 500 high dose FCM infusions [Table 3]. Four hundred and seven (90.4%) patients received a single infusion, 36 (8%) patients had two infusions and seven (1.6%) patients had received three infusions of the study drug. All patients (100%) had a successful administration of the FCM. No patient had immediate hypersensitivity reactions or any other serious AE. One patient was found to have rise in BP to levels of accelerated hypertension after the infusion. The patient was asymptomatic and the BP improved shortly after administration of antihypertensive drugs. In all other patients, no clinical relevant changes in vital signs or physical examinations were noted after FCM infusion. The AEs observed or reported after the infusion in 14 (2.8%) patients were: Accelerated hypertension (0.2%), feeling abnormal (0.6%), body aches and headache (0.6%) each, and infusion site reaction (0.8%). All AE were considered to be mild to moderate in severity. None of the patients had an AE of severe nature.
Efficacy
Two hundred and sixty-one (81.6%) out of a total of 320 patients who were expected for follow up visit by the end of the study period, had a follow up Hb. Due to cost constraints, the repeat iron status evaluation was available in a small proportion (44 [16.9%]) of them. These patients formed the mITT population used for efficacy analysis.
Changes in hemoglobin and hemoglobin categories between baseline and follow up (n = 261)
Two hundred and sixty-one patients had a follow up Hb [Table 4 and Figure 1]. There was a significant increase in the mean Hb after a period of 11 ± 7.2 weeks. The Hb increased from a baseline of 9.1 ± 2.1 g/dl to 10.7 ± 2 g/dl showing an increase of 1.7 ± 1.5 g/dl (95% CI, 1.5-1.9, P = 0.001). This significant increase in Hb was uniformly seen across all stages of CKD. In stage 3 CKD patients, the Hb increased from 9.8 ± 2.2 g/dl to 11.6 ± 2 g/dl showing an increase of 1.8 ± 1.4 g/dl (95% CI, 1.5-2.1, P = 0.001). In stage 4 CKD patients, the Hb increased from 9.6 ± 1.8 g/dl to 11.1 ± 1.9 g/dl showing an increase of 1.5 ± 1.6 g/dl (95% CI 1.2-1.9, P = 0.001). In stage 5 CKD patients, the Hb increased from 8.2 ± 2 g/dl to 10 ± 1.9 g/dl showing an increase of 1.7 ± 1.6 g/dl (95% CI, 1.4-2.1, P = 0.001). In stage 5D CKD patients (17 PD-CKD and two HD-CKD), the Hb increased from 8.3 ± 1.6 g/dl to 9.7 ± 1 g/dl showing an increase of 1.4 ± 1.4 g/dl (95% CI, 0.7-2.1, P = 0.001). In HD-CKD patients, the Hb increased from baseline Hb of 8.6 ± 0.6 g/dl to 9.7 ± 1.1 g/dl showing an increase of 1.2 ± 0.5 g/dl (95% CI,-3.2-5.6, P = 0.188). Sixty-one (48.4%) out of a total of 126 patients with elevated CRP levels had a follow up Hb, which was a significant rise. The Hb increased from a baseline of 9.1 ± 2.3 g/dl to 11 ± 2.1 g/dl showing an increase of 1.8 ± 1.3 g/dl (95% CI, 1.5-2.2, P = 0.001). Two hundred and one (77%) patients were getting ESA, 70 (35%) were on stable doses before and during the study and in others, the initial dose was maintained during the study. The rise in Hb was significant in both subset of patients with ESA at baseline and those with ESA initiated after FCM infusion (P = 0.001). Even the patients not on ESA had a significant rise in their Hb levels (P = 0.001).


- Mean changes from baseline to follow up for Hb. The paired samples T-test was done to compare the mean changes in Hb. *indicates significant difference from baseline, P = 0.001
One hundred and eighty-eight (72%) patients had a rise in Hb of ≥1 g/dl. As compared to the baseline, there was a significant change in the proportion of patients in different Hb categories on follow up [Table 4 and Figure 2]. The proportion of patients in Hb ≤9 and 9.1-10 g/dl categories decreased and proportion of patients in Hb 10.1-11 and >11 g/dl categories increased (P = 0.001). There were 55 (21.1%) patients with Hb ≤9 g/dl as compared to 137 (52.5%) at the baseline. Similarly the number of patients with Hb 9.1–10 g/dl decreased from 45 (17.2%) to 42 (16.1%) at follow up. Whereas as compared to the baseline, the number of patients with Hb 10.1-11 g/dl increased from 37 (14.2%) to 65 (24.9%) and patients with Hb >11 g/dl increased from 42 (16.1%) to 99 (37.9%) at follow up.

- Changes from baseline for percent of subjects in different Hb categories on follow up
Changes in hemoglobin and iron indices between baseline and follow up (n = 44)
There were 44 (16.9%) patients with a follow up Hb and iron status done after a period of 15.1 ± 11.5 weeks [Table 5 and Figure 3]. There was a significant increase in mean values of Hb, TSAT and ferritin on follow up. The Hb increased from a baseline of 9.8 ± 1.7 g/dl to 11.4 ± 2.2 g/dl showing an increase of 1.6 ± 2.2 g/dl (95% CI, 0.9-2.3, P = 0.001). The TSAT increased from baseline of 19.4 ± 7.5% to 28.5 ± 15.8% at follow up showing an increase of 9.1 ± 16.9% (95% CI, 3.8–14.4, P = 0.001). The ferritin levels increased from baseline value of 223 ± 171 ng/ml to 629 ± 458 ng/ml at follow up showing an increase of 406 ± 449 ng/ml (95% CI, 268-545, P = 0.001). Five PD-CKD patients had follow up iron status done. The TSAT increased from baseline of 19.5 ± 14.9% to 25.5 ± 23.3% at follow up showing an increase of 6 ± 30.4% (95% CI,−42.4-54.5, P = 0.719). The ferritin levels increased from baseline value of 324 ± 222 ng/ml to 1030 ± 627 ng/ml at follow up showing an increase of 705 ± 663 ng/ml (95% CI,-118.4-1529, P = 0.076).


- Mean changes from baseline to follow up for Hb, TSAT and ferritin. The paired samples T-test was done to compare the mean changes in Hb, TSAT and ferritin. *indicates significant difference from baseline, P = 0.001
Discussion
Erythropoiesis-stimulating agents and iron form the cornerstone of anemia management in CKD. IV iron supplementation in CKD is effective and has got acceptable safety; it permits replacement of iron stores for erythropoiesis, and improves the responsiveness to ESAs, as well as reduces the requirement for costly ESA therapy and transfusions.[172122]
Until recently, with the exception of iron dextran-containing preparations, the available IV iron compounds were unable to provide high doses of iron as a single administration. Thus, for iron gluconate doses of 62.5-125 mg of iron were the maximum recommended at a single sitting whereas for iron sucrose bolus doses of up to only 200 mg were recommended.[67] Iron dextran can be administered as a single dose, but this requires administration over a period of 4-6 h. In addition, iron dextran complexes can cause fatal dextran-induced anaphylactic reactions.[23] Although iron sucrose also lacks the hypersensitivity, it has to be given in the form of small IV infusions to avoid the release of free iron that can provide a dose of 1000 mg, iron sucrose needs to be given in the form of 5 doses of 200 mg each over 14 days period. FCM is an innovative nondextran iron complex that has been developed for rapid IV administration in high doses. It lacks the hypersensitivity associated with iron dextran and does not require administration of a test dose. With one infusion of FCM, total iron replenishment can be achieved. Thus, total dose infusion with FCM can reduce the number of patient visits and venipunctures and is a more convenient form of IV iron therapy.[8] Such high doses of IV iron are essential when rapid replacement of iron stores is desired or when iron therapy is given along with ESA.[8]
Ferric carboxymaltose was found to be well tolerated in clinical trials. Most AEs noted with FCM were considered to be mild to moderate in severity. The AEs probably related to FCM were found to be numerically higher than placebo and similar to comparators or oral iron.[8] Safety and tolerability of FCM has been demonstrated in 1968 patients with low incidence of AEs, no serious AE and low rates of discontinuation. No evidence of anaphylactic reactions or immunogenic potential was demonstrated during clinical studies.[8] AEs that included ≥1% of patient population included headache (2.5%), nausea (1.7%), injection site reaction (1.6%), dizziness (1%), rash (1.1%), vomiting, upper abdominal pain and diarrhea (1.2%), decreased serum phosphorous (1.5%) and increased alanine transaminase levels (1%).[8] No clinical relevant changes in vital signs or physical examinations were reported. No serious AE that occurred in clinical trials on FCM was likely to be related to treatment with FCM.[824]
Safety and tolerability of IV FCM (15 mg/kg, maximum 1000 mg over 15 min) was confirmed in a crossover, randomized, placebo controlled trial of 559 patients with IDA.[24] During the first 24 h of the treatment period, 9.3% of subjects receiving FCM and 4.8% receiving placebo reported AEs. None was a serious AE.[24] Further, a meta-analysis of 14 studies found that there was little difference in terms of withdrawals and AEs, including serious AEs or hypotension, between FCM and all comparators or oral iron alone.[12]
In ND-CKD patients and in HD-CKD patients, FCM is well tolerated and is associated with few AEs.[17181920] In a phase 3, randomized controlled trial (RCT) involving 147 ND-CKD patients, Qunibi et al. found that 1000 mg of FCM administered over 15 min was better tolerated than oral iron. Total 2.7% of the patients in the FCM group experienced at least one possibly drug-related AE and no AE that were considered to be drug-related were experienced by more than one subject. None of the patients had a serious AE.[18] In another uncontrolled study involving HD-CKD, serious AEs were reported in 12 out of 163 (7.4%) patients treated with IV FCM 100-200 mg, two to three times weekly for ≤6 weeks but no patient had serious AE.[19]
In another RCT involving 204 ND-CKD and 50 HD-CKD patients, FCM in doses of 200 mg for HD-CKD patients and up to 1000 mg in ND-CKD patients were safe and well tolerated.[20] There was a significant difference in the incidence of serious AEs between the standard medical care (SMC) (8.9%) and FCM groups (3.5%), and between the iron sucrose/sodium ferric gluconate subgroup (9.6%) and the FCM group. The most common treatment-emergent AEs in the FCM group were nausea (4.4%), vomiting (2.9%), peripheral edema, arthralgia, dizziness and hypertension (2.0% each).[20] No hypersensitivity reactions were reported during the study.[20]
In the current prospective observational study, 500 high dose FCM infusions were administered to 450 patients. Majority (94%) of the patients was ND-CKD and amongst 27 dialysis patients, majority (78%) was PD-CKD. Administration of a high dose FCM was found to be safe and well tolerated. There was a low incidence (2.8%) of AEs. All AEs were judged to mild to moderate in severity. No patient had immediate hypersensitivity reactions or any other serious AE.
Ferric carboxymaltose has shown efficacy in the treatment of IDA associated with varied indications including inflammatory bowel disease, congestive heart failure, postpartum state, ND-CKD, HD-CKD. In majority of these trials, FCM has been compared in oral ferrous sulfate except one trial in HD-CKD patients where iron sucrose was used as a comparator molecule. In comparison with oral ferrous sulfate, FCM shows faster and at times greater increase in Hb levels and faster replenishment of iron stores. Further the duration of therapy required was much less than oral iron therapy. The efficacy of FCM was found to be comparable to iron sucrose in HD-CKD patients.[1025]
Ferric carboxymaltose was found to be more effective than oral iron in the study by Qunibi et al. A total of 255 ND-CKD patients with IDA receiving stable dose of ESA were randomized to FCM 1000 mg over 15 min or oral ferrous sulfate for 8 weeks In the mITT population, FCM showed higher proportion of patients achieving a Hb increase ≥ 1 g/dl at any time (60.4% vs. 34.7%; P < 0.001). It also increased higher increase in Hb (1 ± 1.1.1 vs. 0.5 ± 1.2 g/dl; P = 0.005), higher increase in ferritin (432 ± 189 vs. 18 ± 45 ng/ml; P < 0.001) and higher increase in TSAT (13.6 ± 11.9% vs. 6.1 ± 8.1%; P ≤ 0.001).[18]
A single dose of FCM up to1000 mg in ND-CKD patients showed comparable efficacy with other IV iron formulations requiring multiple infusions. There were no statistically significant differences between the FCM and SMC groups in indices of Hb improvement, including proportions of patients achieving a ≥1 g/dl increase in Hb and proportions of patients achieving Hb level of >12 g/dl. A total of 27.2% patients showed Hb increase of ≥1 g/dl at the end of 4 weeks. There was an increase 0.6 ± 0.9 g/dl from baseline Hb of 10.4 ± 0.9 g/dl at end of 4 weeks. There was a significant increase in iron indices at 4 weeks from baseline in ND-CKD and HD-CKD patient, TSAT increased from 20.7 ± 8.8 to 29.3 ± 10.8% and ferritin levels increased from a baseline of 123 ± 111 to 366 ± 181 ng/ml.[20]
In another phase II uncontrolled study, IV FCM (100-200 mg, two to three times weekly for ≤6 weeks; mean 2133 mg FCM) in 162 anemic HD-CKD patients, FCM was shown to be effective in the correction of Hb levels and iron stores. In the ITT analysis, an Hb increase ≥1 g/dl was observed in 100 of 162 (61.7%) patients, and mean Hb levels increased from 9 ± 1.3 g/dl at baseline to 10.3 ± 1.6 g/dl at follow up (10 weeks), ferritin from 67 to 334 ng/ml, and TSAT from 17 to 31%.[19]
In the present study, administration of a single high dose FCM led to a significant improvement in Hb and iron status. Efficacy analyses of our study found that high dose FCM resulted in a significant increase in the mean Hb on first follow up at 11 ± 7.2 weeks. This significant increase in Hb was uniformly seen across all stages of CKD. Majority (72%) of the patients had an increase in Hb of ≥1 g/dl. As compared to the baseline, there was a significant change in the proportion of patients in different Hb categories on follow up. The proportion of patients in Hb ≤9 and 9.1-10 g/dl categories decreased and proportion of patients in Hb 10.1–11 and >11 g/dl categories increased (P = 0.001). Further, nearly 38% of patients were having an Hb >11 g/dl after a single high dose FCM. Patients with an elevated CRP levels also had a significant increase in Hb Levels. The excellent improvement in Hb seen is compounded by the factor that a high proportion (77%) of the patients was on ESAs but the ESA dose remained stable during the study. The rise in Hb was significant in both subsets of patients with ESA at baseline and those with ESA initiated after FCM infusion (P = 0.001). Even the patients not on ESA had a significant rise in their Hb levels (P = 0.001). A higher percentage (72%) of patients experiencing an increase in Hb in our study can explained by the fact that majority of the patients were getting ESA and had a longer duration of follow up as compared to the other studies discussed above. The patients were treated as per KDIGO guidelines in the real world clinical settings outside the RCTs. ESA were used keeping in view of the patient's Hb concentration, and clinical circumstances, avoidance of red cell transfusion, the presence of symptoms attributable to anemia and to improve the quality of life.
The single high dose FCM corrects the iron deficiency and leads to significant improvement in iron stores. Though due to cost constraints the repeat iron status evaluation was available in a small proportion of the mITT population for efficacy analysis, but the results showed a significant improvement in Hb and iron status at 15.1 ± 11.5 weeks. The Hb increased from a baseline of 9.8 ± 1.7 g/dl to 11.4 ± 2.2 g/dl showing an increase of 1.6 ± 2.2 g/dl (95% CI, 0.9–2.3, P = 0.001). The TSAT increased from baseline of 19.4 ± 7.5% to 28.5 ± 15.8% at follow up showing an increase of 9.1 ± 16.9% (95% CI, 3.8–14.4, P = 0.001). The ferritin levels increased from baseline value of 223 ± 171 ng/ml to 629 ± 458 ng/ml at follow up showing an increase of 406 ± 449 ng/ml (95% CI, 268–545, P = 0.001). The iron status improved in PD-CKD patients but it was not statistically significant perhaps due to number of these patients being small.
The study results confirm the safety and efficacy of the FCM demonstrated by the earlier RCTs. A large dose of FCM (1000 mg) administered as a single bolus dose is well tolerated and has low incidence of AE. The limited data on repeat iron in the study indicates that a high dose FCM leads to significant improvement in iron stores and may corrects the iron deficiency. Further, considering the extent of improvement in iron status, a 6 monthly high dose FCM may be enough to maintain adequate iron stores in ND-CKD and PD-CKD patients.
High dose IV FCM represents a viable treatment option for IDA in ND-CKD and PD-CKD patients. It is safe and effective in restoring iron stores, and may potentially save time and improve patient adherence. The most obvious benefit for both the patient and the health care team is the need for fewer IV iron administrations, and this may have a considerable impact on patients who live some distance from the treatment center. Additionally, a fewer IV cannulations may be beneficial in terms of future arteriovenous fistula creation and survival. Although no formal health economic assessment was performed, it is likely that this would be positive, particularly for patients requiring traveling considerable distances.
Conclusion
In conclusion, FCM can be rapidly administered. A high dose administration of FCM was safe and well tolerated. It was effective in the treatment of iron deficiency in ND-CKD and PD-CKD patients. It may be the IV iron of choice in treatment of CKD anemia resulting in considerable savings in time and cost. High dose FCM administration is convenient and optimal form of IV iron therapy that is most practical in ND-CKD and PD-CKD patients.
Source of Support: Nil
Conflict of Interest: None declared.
References
- Iron status and hemoglobin level in chronic renal insufficiency. J Am Soc Nephrol. 2002;13:2783-6.
- [Google Scholar]
- Iron supplementation to treat anemia in patients with chronic kidney disease. Nat Rev Nephrol. 2010;6:699-710.
- [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for Anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279-335.
- [Google Scholar]
- Clinical use of intravenous iron: Administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program 2010 2010:338-47.
- [Google Scholar]
- Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J Nephrol. 2006;19:161-7.
- [Google Scholar]
- Ferrlecit Prescribing Information. Available from: http://www.products.sanofi.us/ferrlecit/ferrlecit.html
- [Google Scholar]
- Venofer Prescribing Information. Available from: http://www.venofer.com/PDF/Venofer_IN2340_Rev_9_2012.pdf
- [Google Scholar]
- Ferric carboxymaltose: A review of its use in iron-deficiency anaemia. Drugs. 2009;69:739-56.
- [Google Scholar]
- The new generation of intravenous iron: Chemistry, pharmacology, and toxicology of ferric carboxymaltose. Arzneimittelforschung. 2010;60:345-53.
- [Google Scholar]
- Pharmacokinetics, safety and tolerability of intravenous ferric carboxymaltose: A dose-escalation study in volunteers with mild iron-deficiency anaemia. Arzneimittelforschung. 2010;60:362-72.
- [Google Scholar]
- Pharmacodynamics and safety of ferric carboxymaltose: A multiple-dose study in patients with iron-deficiency anaemia secondary to a gastrointestinal disorder. Arzneimittelforschung. 2010;60:373-85.
- [Google Scholar]
- Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 2011;11:4.
- [Google Scholar]
- A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: The ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182-92.
- [Google Scholar]
- FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846-853e1.
- [Google Scholar]
- Large-dose intravenous ferric carboxymaltose injection for iron deficiency anemia in heavy uterine bleeding: A randomized, controlled trial. Transfusion. 2009;49:2719-28.
- [Google Scholar]
- Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: A randomized controlled trial. Obstet Gynecol. 2007;110:267-78.
- [Google Scholar]
- The efficacy of a single dose of intravenous ferric carboxymaltose (Ferinject) on anaemia in a pre-dialysis population of chronic kidney disease patients. J Ren Care. 2009;35:18-23.
- [Google Scholar]
- A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26:1599-607.
- [Google Scholar]
- The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: A multi-centre, open-label, clinical study. Nephrol Dial Transplant. 2010;25:2722-30.
- [Google Scholar]
- Intravenous ferric carboxymaltose versus standard medical care in the treatment of iron deficiency anemia in patients with chronic kidney disease: A randomized, active-controlled, multi-center study. Nephrol Dial Transplant. 2013;28:953-64.
- [Google Scholar]
- Iron supplementation in the non-dialysis chronic kidney disease (ND-CKD) patient: Oral or intravenous? Curr Med Res Opin. 2010;26:473-82.
- [Google Scholar]
- Iron therapy in chronic kidney disease: Current controversies. J Ren Care. 2009;35(Suppl 2):14-24.
- [Google Scholar]
- National Institute for Health and Clinical Excellence: Clinical Guideline 39: Anaemia Management in People with Chronic Kidney Disease (CKD) 2006. Available from: http://www.guidance.nice.org.uk/CG39
- [Google Scholar]
- Safety and tolerability of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Hemodial Int. 2010;14:47-54.
- [Google Scholar]
- The efficacy and safety of current intravenous iron preparations for the management of iron-deficiency anaemia: A review. Arzneimittelforschung. 2010;60:399-412.
- [Google Scholar]







