Translate this page into:
Frequency of kidney diseases and clinical indications of pediatric renal biopsy: A single center experience
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Kidney biopsy occupies a fundamental position in the management of kidney diseases. There are very few renal pathology studies available in the literature from developing world. This study scrutinized the frequency and clinicopathological relationship of kidney biopsies done at the kidney center from 1997 to 2013 amongst pediatric patients. Kidney allograft biopsy were excluded. The specimen was examined under light microscopy and immunofluorescence while electron microscopy was not done. The study includes 423 patients, mean age was 10.48 ± 4.58 years, males 245 (57.9%) were more than females 178 (42.1%). Nephrotic syndrome 314 (74.2%) was the most common clinical presentation followed by acute nephritic syndrome 35 (8.3%) and acute renal failure 24 (5.7%). Primary glomerulonephritis (PGN) was the most common group of diseases, seen in 360 (85.1%) followed by secondary glomerulonephritis (SGN) in 27 (6.4%) and tubulointerstitial nephritis in 21 (5.0%). Among PGN, minimal change disease (MCD) was the most dominant disease, with 128 (30.3%) cases followed by focal segmental glomerulosclerosis FSGS in 109 (25.8%) and membranous glomerulonephropathy in 27 (6.4%). Lupus nephritis (LN) was the leading cause of glomerular disease in SGN followed by hemolytic uremic syndrome. In conclusion, MCD is the most common histological finding, especially in younger children and FSGS is second to it. SGN is rare, and the most common disease in this category is LN while tubulointerstitial and vascular diseases are infrequent.
Keywords
Children and renal diseases
epidemiology
nephrotic syndrome
renal pathology
Introduction
Kidney biopsy has a cardinal role in the management of kidney diseases. Biopsy proven kidney diseases impart valuable information about incidence, distribution and possible control of disease with effective and directed treatment. Kidney biopsy in children is a real challenge since the early days of biopsy, due to uncooperative patient with small and mobile kidneys. Expectedly, many innovations were done over the time in localization of kidneys and biopsy technique particularly in children.[12345] Now, the procedure in children is regarded safe and advisable.[5] Therefore, now more biopsies are performed even in younger children as compared with the past.
There is great variation in epidemiology of renal histopathology all over the world, even within a region, due to differences in race, socioeconomical difference and policy of performing kidney biopsy in children. This was the reason available; data from developing countries are unorganized except in Japan and Korea.[678910111213141516171819202122]
This is a retrospective analysis of pediatric renal biopsy performed in a single center tertiary kidney hospital. The purpose of this retrospective analysis is to describe the epidemiology of biopsy-proven kidney disease in Pakistani pediatric population.
Materials and Methods
This study includes all pediatric patients of <18 years of age, who had percutaneous kidney biopsy between 1997 and 2013. Patients with kidney graft biopsy were excluded. Over the span of 17 years, there were considerable variations in the way that the children were prepared, and the technique employed for kidney biopsy. Different techniques of biopsies were used: In the early days, biopsies were done under sedation with Tru-cut biopsy needle; later on fluoroscopy with a biopsy gun was used for short period of time. In the last 10 years, biopsies were done under anesthesia, with automated gun along with real-time ultrasound guidance. All biopsies were done by a consultant or registrar nephrologist. Two cores of kidney tissues were taken and sent to pathological laboratory. A sample was considered adequate if more than eight glomerular were present. The specimen was examined only under light microscopy and immunofluorescence, as electron microscopy is not available at our center.
The indications of renal biopsy incorporate nephrotic syndrome (NS), nephritic syndrome, and unexplained kidney failure with normal size kidneys, hematuria, acute kidney failure, chronic kidney disease (CKD), asymptomatic urinary abnormalities, and rapidly progressive glomerulonephritis (RPGN). Among patient who had idiopathic NS, we performed biopsies only if the age of patients were <12 months, or more than 17 years if the patient had steroid resistant NS and steroid dependent NS.
We defined NS if the patient had urinary protein excretion of >40 mg/m2 in a 24 h urine collection, or spot protein excretion ratio of >2 mg/mg with generalized body swelling and hypoalbuminemia.
Steroid resistant was defined if there was persistent proteinuria of more than 40 mg/m2/h after 4 weeks of prednisolone treatment. Steroid dependent NS was defined as two relapses during steroid treatment or within 2 weeks after completing the treatment. Frequently relapsing NS was defined if there were two or more relapses in 6 months or 4 or more relapses in 12 months duration.
Renal disease were classified in five groups (1) primary glomerulonephritis (PGN), (2) secondary glomerulonephritis (SGN), (3) tubulointerstitial nephritis (TIN), (4) hereditary glomerular nephritis and (4) miscellaneous.
Primary renal disease encompassed minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), membranous glomerulonephropathy (MGN), mesangioproliferative glomerulonephritis (MesPGN), membranoproliferative glomerulonephritis (MPGN), chronic glomerulonephritis, postinfectious (PIGN), IgA nephropathy (IgAN), SGN included, lupus nephritis (LN), amyloidosis, hemolytic uremic syndrome (HUS), tubulointerstitial diseases include acute and chronic TIN, acute tubular necrosis (ATN) and oxalosis. Hereditary nephritis included Alport Syndrome and congenital NS.
Statistical analysis
Data were analyzed using SPSS version 17.0. SPSS V.17.0.0.0 (SPSS Inc. Chicago IL); All categorical variables were described as frequency and percentage, and all continuous variables were reported as mean with standard deviation.
Results
The study includes 423 patients, with slight male dominance (57.9%) and mean age 10.48 ± 4.58 (range 0.1-17) years.
The numbers of biopsy performed increased gradually but not steadily, in fact, it was low during 2001–2007 due to obstacles in regular availability of pediatric nephrologist in this period [Figure 1].
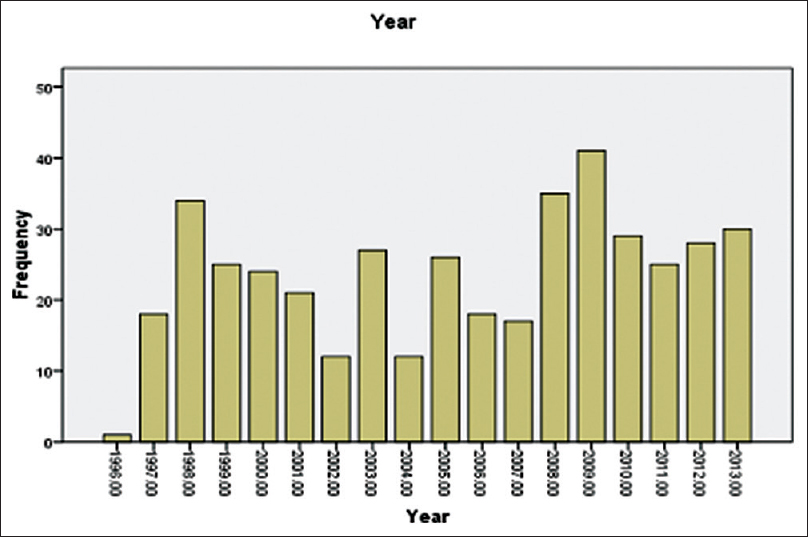
- Frequency of renal biopsy done over span of 17 years
Nephrotic syndrome was the most common clinical presentation for which kidney biopsy was done, followed by acute nephritic syndrome in 35 cases (8.3%), acute renal failure in 24 (5.7%), isolated hematuria in 20 (4.7%), RPGN in 16 (3.8) and CKD in 14 (3.3%) [Figure 2].

- Clinical presentation
The clinicopathological category of glomerulonephritis showed that the PGN is the most common disease with 360 cases (85.1%) followed by SGN in 27 (6.4%), TIN in 21 (5.0%), hereditary kidney disease in 7 (1.7%) while 8 (1.9%) biopsies were inadequate [Figure 3].

- Major classification of renal diseases
Among PGN, MCD is the most dominant disease in our group, 128 (30.3%) followed by FSGS 109 (25.8%), MGN 27 (6.4%), PIGN 20 (4.7%), MesPGN 20 (4.7%), IgAN was relatively low 9 (2.1%). LN 20 (4.7%), was the leading cause of glomerular disease in SGN followed by HUS 5 (1.2%) and amyloidosis 2 (0.5%) [Table 1].
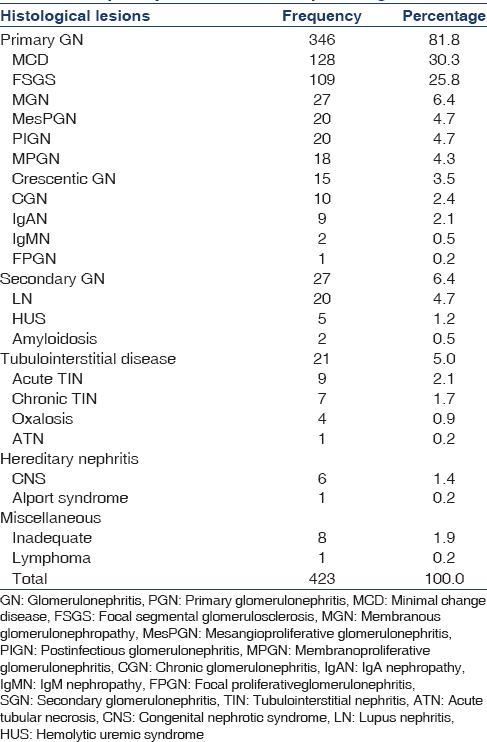
Considering tubulointerstitial disease, acute TIN was found in 9 (2.1%) cases, chronic TIN 7 in (1.7%), and oxalosis in 4 (0.9%). Due to unavailability of electron microscope, a confirmed diagnosis of hereditary diseases was not possible. Congenital NS was found in 6 (1.4%) [Table 1].
Table 2 depicts the age and gender relationship with some of the important glomerular diseases; it is interesting to see that all primary glomerular diseases are predominant in males, while in secondary glomerular diseases LN is exceptionally high among female (95 vs 5%). The other important observation is that the MCD was more common in younger children of age up to 10 years (72.65% and 27.35%). However, as the age advances from childhood to adolescent the incidence of FSGS exceeds that of MCD.

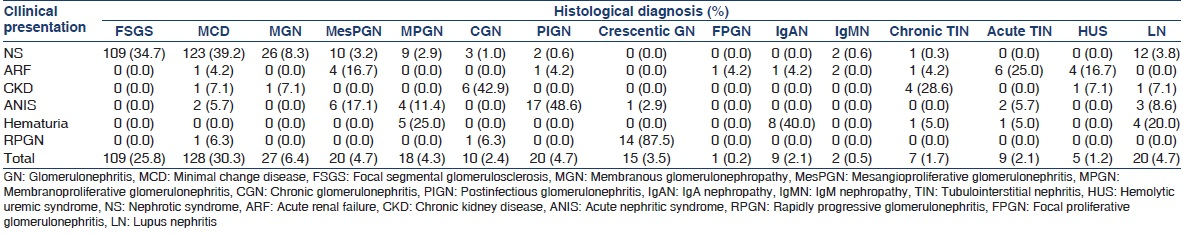
Most primary and secondary glomerular diseases presented with NS, on the other hand acute TIN and HUS presented with acute kidney failure, while chronic glomerulonephritis (GN) and chronic TIN mostly presented with CKD and all crescentic GN presented with RPGN [Table 3].
Discussion
This study lays out some fact and figures about the epidemiology and clinicopathological correlation of children biopsy performed in a tertiary care kidney hospital in a metropolitan city of Pakistan. As noted, there is male predominance not only in overall population of the study but also in all type of primary and secondary glomerular diseases except in LN which is exclusively a female disease.[11121920]
In this study, NS was found to be the most common indication of renal biopsy that is akin to most of the studies.[11121314] Among NS, MCD was the most common pathological lesion followed by FSGS, which is also consistent with most of the regional studies.[111213141920]
In the last two decades, there has been change in glomerular disease pattern in adults, and FSGS emerges as the most frequent lesion over MCD and MGN.[2324] The same changing pattern in children was also observed but, the reason of this changing pattern is less clear.[8920222526] We think that this is a reflection of the differences in the mean age of the participant of the different cohorts. For example Gulati, first reported an increasing incidence of FSGS,[9] over MCD, particularly in adolescents.[25] MCDwas most frequent in children between 1 and 8 years.[21] Same observation was made by Mubarak and Kazi.[27] It is difficult to comment on reports of Iran[20] and Saudi Arabia[22] because both studies did not mention the mean and median of age of the study group. In the North American population, predominance of FSGS can be explained by ethnicity and age as well.[26]
The second most common lesion in our study was FSGS, which is very close to minimal change nephropathy. The median age of our population was 11 years and mode was 14 years, relatively more adolescents were included. In contrast, the population of Hafez,[10] Moorani[12] and Absar has lower average age, so the proportion of MCD is high as compare with FSGS [Table 4].

The incidence of membrane nephropathy in children is reportedly low in another part of the world ranging from 0.3% to 4.4%.[1620] Our population comprises 6.4% of membrane nephropathy that is comparable to the local and international data [Table 4].
Mesangioproliferative GN was found in 4.7% of the population, which is in consistent with other regional data[1128] although Hafeez and Akhtar, from the same country, reported a very high incidence (34.2% and 17.83%). Mesangioproliferative GN showed a high variability in reporting, it ranges from as low as 0.8% by Morrani[12] from Pakistan to as high 27.7% in Southern Croatia[29] and 33% in Jordan.[30] This variation could be due to inability of the center to perform immunofluorescence, as it emerges out from both of studies conducted in Pakistan.[1013] The other reasons could be the differences in the histopathological criteria that a center used to describe the mesangioproliferative lesion. As we noted, for example, in Croatian[29] and Jordanian[30] studies, the presence of IgM and C3 with mesangial hypercellularity was reported as mesangioproliferative GN, while it qualifies the criteria of IgM Nephropathy by other centers.[11]
The incidence of IgAN is mostly due to differences in policies and practices of performing kidney biopsies, for example in western and eastern Europe and in Japan it is leading cause of GN.[171819] On the other hand, it is reportedly low in India,[15] Iran[20] and Pakistan.[11] It is difficult to comment on the incidence of IgAN in Pakistan due to two reasons. Firstly, most of the studies done in the past were without immunofluorescence.[1013] Secondly, reporting biases are found. Mubarak[31] after analyzing local studies, showed concern about the undiagnosed high incidence of IgAN in Pakistan. Ironically, Lanewala and Mubarak, from the same center reported low incidence of IgAN 1.6%.[22] Muzzafar[32] reported a high incidence of IgAN 12.65% but Absar et al.[14] and from the same center during the same period, in pediatric population reported not a single case of IgAN.
Among the SGN, LN was found almost exclusively in female. The female preponderance is well-known. Our result shows similar prevalence with other regional studies.[1124]
None of the studies from Pakistan reported congenital NS while 1.4% of our biopsy samples constituted congenital nephrotic syndrome of Finnish variety. This might be due to policy of performing kidney biopsy at our center due to availability of expertise. Other congenital diseases were not possible to diagnose due to unavailability of electron microscope. The only case of Alport Syndrome reported on light microscopy due to classical clinical symptom and strong family history.
Tubulointerstitial disease is found to be an uncommon biopsy finding. Our study also showed a marginal occurrence of TIN. The most frequent lesion in our study was acute TIN.
In conclusion, primary glomerular diseases are most common finding in pediatric kidney biopsy and among the entire histological lesion MCD is the commonest, followed by FSGS. Tubulointerstitial, vascular and secondary glomerular diseases are infrequent.
Acknowledgment
We acknowledged Dr. Komal motwani and Mr. M. Ali Qureshi for their help in collecting data preparing and arranging the manuscript.
Source of Support: Nil
Conflict of Interest: None declared.
References
- A modified technique for percutaneous needle biopsy of the kidney. J Pediatr. 1967;70:81-6.
- [Google Scholar]
- Ultrasonics in renal biopsy: An aid to determination of kidney position. Lancet. 1961;2:750-1.
- [Google Scholar]
- The outcome of percutaneous renal biopsy in children: An analysis of 120 consecutive cases. Pediatr Nephrol. 1990;4:600-3.
- [Google Scholar]
- Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol. 2012;7:1591-7.
- [Google Scholar]
- Percutaneous renal biopsy in children: Survey of pediatric nephrologists in Japan. Pediatr Nephrol. 1999;13:693-6.
- [Google Scholar]
- Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis. 1999;34:646-50.
- [Google Scholar]
- Renal biopsy in childhood nephrotic syndrome. J Coll Physicians Surg Pak. 2002;12:454-7.
- [Google Scholar]
- Pattern of pediatric renal disease observed in native renal biopsies in Pakistan. J Nephrol. 2009;22:739-46.
- [Google Scholar]
- Histopathological pattern in childhood glomerulonephritis. J Pak Med Assoc. 2010;60:1006-9.
- [Google Scholar]
- Histological pattern of paediatric renal diseases in northern Pakistan. J Pak Med Assoc. 2011;61:653-8.
- [Google Scholar]
- Ten year experience of pediatric kidney biopsies from a single center in Pakistan. Indian J Nephrol. 2010;20:190-2.
- [Google Scholar]
- Indications and results of renal biopsy in children: A 10-year review from a single center in Serbia. J Nephrol. 2012;25:1054-9.
- [Google Scholar]
- Pattern of glomerular diseases in Sudanese children: A clinico-pathological study. Saudi J Kidney Dis Transpl. 2010;21:778-83.
- [Google Scholar]
- British Association of Paediatric Nephrology. Renal biopsies in children: Current practice and audit of outcomes. Nephrol Dial Transplant. 2010;25:485-9.
- [Google Scholar]
- Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1,850 biopsied cases. Research Group on Progressive Chronic Renal Disease. Nephron. 1999;82:205-13.
- [Google Scholar]
- Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children).Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology. Nephrol Dial Transplant. 1998;13:293-7.
- [Google Scholar]
- Glomerular diseases in Iranian children: Clinico-pathological correlations. Pediatr Nephrol. 2003;18:925-8.
- [Google Scholar]
- Histopathological spectrum of childhood nephrotic syndrome in Indian children. Pediatr Nephrol. 2003;18:657-60.
- [Google Scholar]
- Changing trends of histopathology in childhood nephrotic syndrome in western Saudi Arabia. Saudi Med J. 2002;23:317-21.
- [Google Scholar]
- Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: A 20-year renal biopsy study. Am J Kidney Dis. 1995;26:740-50.
- [Google Scholar]
- Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis. 1997;30:621-31.
- [Google Scholar]
- Spectrum of adolescent-onset nephrotic syndrome in Indian children. Pediatr Nephrol. 2001;16:1045-8.
- [Google Scholar]
- Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999;55:1885-90.
- [Google Scholar]
- Histopathological spectrum of childhood nephrotic syndrome in Pakistan. Clin Exp Nephrol. 2009;13:589-93.
- [Google Scholar]
- Childhood renal diseases in Korea. A clinicopathological study of 657 cases. Pediatr Nephrol. 1987;1:664-9.
- [Google Scholar]
- Epidemiology of renal disease in children in the region of southern Croatia: A 10-year review of regional renal biopsy databases. Med Sci Monit. 2007;13:CR172-6.
- [Google Scholar]
- The spectrum and outcome of primary glomerular disorders in 146 Jordanian children. Int Pediatr. 2002;17:239-42.
- [Google Scholar]
- The prevalence of IgA nephropathy in Pakistan: Only a tip of the iceberg. J Pak Med Assoc. 2009;59:733.
- [Google Scholar]
- The frequency of IgA nephropathy at a single center in Pakistan. J Pak Med Assoc. 2003;53:301-5.
- [Google Scholar]







