Translate this page into:
Pegylated interferon monotherapy for hepatitis C virus infection in patients on hemodialysis: A single center study
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
There is no published study from India on hepatitis C virus (HCV) treatment in dialysis patients. Patients on dialysis with HCV infection treated with pegylated interferon (Peg-INF) monotherapy were studied. All patients were subjected to HCV-polymerase chain reaction, viral load, genotype, and liver biopsy. Quantitative HCV-RNA was performed monthly. Patients with genotype 1 and 4 were given 12 month therapy while those with genotypes 2 and 3 were given 6 months therapy. Response was classified as per standard criteria of rapid virological response (RVR), early virological response (EVR), end of treatment response (ETR), and sustained virological response (SVR). A total of 85 patients were treated. Mean age was 35.2 ± 10.5 (range 15–67) years, and 77.6% were males. HCV genotypes were 1 in 40.9%, 2 in 12%, 3 in 36.1%, 4 in 3.6%, and others in 7.2%. Mean viral load was 106 copies/mL. Mean liver biopsy grade was 4 ± 1.7 and stage 0.8 ± 0.8. Mean time from diagnosis of HCV infection and the treatment start was 10.7 ± 14.3 months. One patient died of unrelated illness, one was lost to follow-up, and three could not sustain treatment due to cost. Forty-three of the 80 (54%) patients had RVR while 49 (61%) patients had EVR and ETR. There was no difference in term of RVR related to genotype. Fifty -four percentage had SVR. Mild flu-like symptoms were seen in all patients. Sixty-four (80%) patients required increase in erythropoietin doses. Twenty-eight (35%) patients developed leukopenia (three treatment-limiting) and 16 (20%) developed thrombocytopenia (one treatment-limiting). Five patients developed tuberculosis, five bacterial pneumonia, and one bacterial knee monoarthritis. None of the patients developed depression. Our study concludes that Peg-INF monotherapy resulted in 54% RVR and SVR in dialysis patients with HCV infection. Therapy was well-tolerated with minimal side effects. There was no effect of viral genotype on response to therapy.
Keywords
Hemodialysis
hepatitis C virus
India
pegylated interferon
Introduction
Hepatitis C virus (HCV) infection is the most common hepatotropic viral infection affecting the patients on maintenance hemodialysis (MHD). Its prevalence in patients on MHD ranges from 6% to 60% worldwide. In India, various studies showed prevalence of HCV in hemodialysis from 12% to 45%.[1] From our own center, we had published prevalence of HCV in MHD to be from 4.3% to 42% depending upon vintage of MHD in our unit; 42% being at the end of MHD, just before renal transplantation (RT).[2] The major factors affecting the HCV incidence during MHD are nosocomial transmission and transmission through blood and blood component.[34] With the development of universal screening of the blood and blood products, nosocomial transmission remains the major route of spread in these patients. We have also shown that chronic liver disease is cause of mortality in RT in the second decade in 25% of the patients at our centers.[5] HCV also increases the risk of serious infection in renal transplant patients.[6] With significant increase in spread of HCV infection in many dialysis units in India, we suggested to isolate HCV-infected patients in addition to universal precaution.[7] This was more relevant, once we found that hospital staff was not strictly following universal precautions.[8]
However, whatever precautions are taken to contain HCV infection during MHD, a significant proportion of patients do develop new HCV infection. KDIGO guidelines had suggested that HCV-infected kidney transplant candidates be considered for treatment with standard interferon (IFN) before transplantation, though the evidence for recommendation was weak.[9] Uncontrolled trials have demonstrated that administration of conventional IFN therapy to patients on MHD with HCV infection achieves sustained virological response (SVR) in about 40% of the cases[101112] and this SVR achieved during MHD is sustained in 80–90% of the recipients following transplantation.[131415] A large retrospective study of HCV infection during MHD had shown that without treatment, there is significantly increased risk for chronic allograft nephropathy.[16] Thus, large bodies of evidence suggest that HCV-infected patients during MHD should be treated before transplantation. A multicenter trial on treatment of HCV in MHD using conventional INF was prematurely terminated because of significant side effects.[17] In non-chronic kidney disease (CKD) patients, pegylated-INF (Peg-INF) with ribavirin has been standard of care. However, KDIGO guidelines suggested conventional INF for treatment of these patients. These guidelines were published in 2008 and until that time, there was limited number of studies on Peg-INF,[1819202122] which might have resulted such recommendation. Our initial experience with conventional INF was not good as patients could not tolerate conventional INF (unpublished data). Following that, we started using Peg-INF monotherapy in these patients. There is no published study from India on therapy of HCV in MHD patients, and thus we thought of sharing our experience on Peg-INF monotherapy in the treatment of HCV during MHD in this part of world.
Methods
All the patients of end-stage renal disease taken for renal replacement therapy at out hospital with HCV infection and treated with Peg-INF monotherapy were included in the present study. At the time of accepting the patient for MHD, anti-HCV was tested in each patient. Any patient who was positive for HCV was taken for MHD in isolated room. During MHD, all patients were monthly tested for anti-HCV, aspartate transaminase, and alanine transaminase (ALT). Patients who had high ALT but negative for anti-HCV were also isolated unless there is satisfactory cause for high ALT.[23] Patients having positive anti-HCV were further investigated with qualitative HCV-RNA, HCV viral load, and HCV-genotype. All patients were subjected to liver biopsy except those patients who had liver cysts associated with polycystic kidney disease or those patients who were on continuous ambulatory peritoneal dialysis. Patients before starting treatment were also tested for hemoglobin (Hb), total leukocyte count (TLC), differential leukocyte count (DLC), platelets, reticulocyte counts, glucose tolerance test, T3 and T4, thyroid stimulating hormone (TSH), anti-thyroid antibodies, anti-thyroid peroxidase antibodies, anti-liver kidney microsomal type 1, anti-mitochondrial antibody, antinuclear antibodies, serum iron, ferritin, transferrin saturation, and alpha-fetoprotein using standard methods. Timing of HCV infection was taken from the time since ALT was high above normal limit or anti-HCV was positive, whichever was earlier. Time in months was also assessed from the time of HCV infection and the time when treatment was started.
HCV infection was diagnosed using the third generation ELISA test kit (J Mitra and Co., Ltd., India). HCV-RNA was determined by real-time polymerase chain reaction (PCR) and involves sequence-specific amplification. This was done on real-time PCR using TaqMan method. HCV genotyping was determined by PCR and involves sequence-specific amplification. Analysis was based on PCR of the core region with the genotype-specific primers, which allows for the determination of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. Each test was performed with positive and negative controls. The PCR products were analyzed using electrophoresis and Gel Doc Systems. For viral load, the amplified product was detected via fluorescent dyes, which was linked to oligonucleotide probes, which bind specifically to the amplified product. Monitoring the fluorescence intensities during the PCR run allows the detection and quantification of the accumulation product, which was monitored on the desktop of computer. Nucleic acid extraction columns (QIAGEN Hamburg), and internal controls were added to lyse buffer to monitor and check for PCR. PCR Master Mix HCV RG RT-PCR reagents – QIAGEN Hamburg were used. Ten microliters of RNA and 15 μL of Master Mix were added to 0.2 mL tubes and loaded onto the real-time PCR machine, Artus 3000TM.
The first two injections of the Peg-INFα2a were given in the dose of 90 µg subcutaneously on nonvascular access forearm of the patient. Paracetamol in 500 mg dose was advised one after the injection and one after 6 h routinely. After that, paracetamol was advised as per the need of the patient. Peg-INF was given on nondialysis days. Before the next dose of injection, test for serum bilirubin, ALT, Hb, TLC, DLC, and platelets were done. Dose of Peg-INF was decreased to 50% if TLC was between 3000/cmm and 4000/cmm, platelets between 50,000/cmm and 75,000/cmm. Dose of the drug was missed if TLC was <3000/cmm and platelets <50,000/cmm. Patients were temporarily treated with granulocyte colony stimulating factors for 1–2 doses for managing leukopenia. Dose of Peg-INF was usually not changed on the basis of Hb value alone, although Hb value was taken into account for increasing the dose of erythropoietin and/or advising blood transfusion. Quantitative HCV-RNA was done every month. If the patient completed the therapy, HCV-RNA was repeated 2 months after the last dose of Peg-INF. After the transplant, RNA was again tested after 3–4 months to classify SVR. Response to therapy was classified as rapid virological response (RVR), early virological response (EVR), end of treatment response (ETR), and SVR based on conventional criteria. After initial 50 patients experience, any patient who did not respond after 3 months of treatment, therapy was stopped. Patients with genotype-1 and 4 were given 12 months therapy while genotype-2 and 3 were given 6 months therapy.
Results
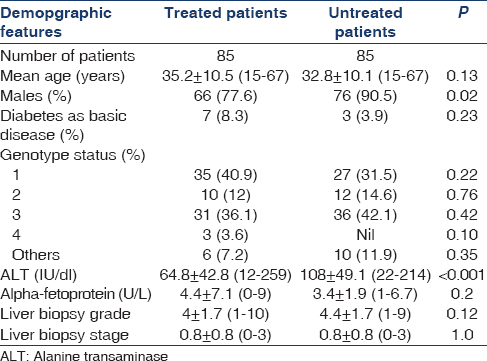
From 2005, 170 patients were screened for possible treatment, of which only 85 patients were initiated on treatment due to affordability. Table 1 shows basic details of treated and untreated patients. The only significant differences between two groups were that males were less common and ALT was significantly lower in treated group as compared to untreated group. In the treated patients, mean age was 35.2 ± 10.5 (15–67) years and males were 77.6%. Diabetes as basic disease was in 8.3% cases. Mean ALT value was 64.8 ± 42.8 (12–259) IU/dl and mean alpha-fetoprotein was 4.4 ± 7.1 (0–9) IU/dl. HCV genotypes were 1 in 40.9%, 2 in 12%, 3 in 36.1%, 4 in 3.6%, and others were in 7.2%. Mean viral load was 106 copies/mL. Liver biopsy grade was 4 ± 1.7 (1–10) and stage 0.8 ± 0.8 (0–3). Details of liver biopsy in our set-up of patients had been published elsewhere.[24] None of the patients had raised bilirubin and/or clinical hepatitis at presentation. Other investigations of patients done before start of treatment are shown in Table 2. Mean time duration from the time of known HCV infection and the treatment start was 10.7 ± 14.3 months (range 3–98). Flow of patient treatment is shown in Figure 1. Of 85 patients started on treatment, 1 patient died of unrelated illness, one lost to follow-up, and three could not sustain treatment due to cost involved. Eighty patients could complete at least 1 month of therapy and thus were analyzed for efficacy of treatment. Forty-three (54%) patients had RVR while 49 (61%) patients had EVR and ETR. There was no difference in term of RVR related to genotype; of the 43 patients, who had RVR, 40% were genotype-3 and 37.5% were genotype-1. None of the patients had a breakthrough infection while on therapy.


- Flow of patients in the study
Mild flu-like symptoms with varying severity were seen in almost all patients. However, regular paracetamol as per our treatment protocol could control these. Some patients did require paracetamol for 2–3 days following initial injections. The majority of the patients, that is, 64 (80%) required increase in erythropoietin doses with or without injectable iron therapy. Maximum dose of erythropoietin given was 14,000 units/week in 3 patients. Twenty-eight (35%) patients developed leukopenia, of which three had treatment-limiting leukopenia. Sixteen (20%) patients developed thrombocytopenia, of which one had treatment-limiting thrombocytopenia. One patient developed optic atrophy during treatment and 1 patient developed lupus activation with cerebritis while on therapy.[25] Six patients who had high TSH required concomitant thyroxin while on Peg-INF. None of the patients who had isolated high anti-thyroid antibodies, anti-thyroid peroxidase antibodies without increased TSH required thyroxin while on Peg-INF. One patient, who had positive ANA, did not have any symptoms of lupus clinically while on therapy. Five patients developed tuberculosis, five bacterial pneumonia, and 1 patient developed bacterial knee monoarthritis. None of the patients developed depression and no patient had treatment-limiting asthenia. Of the treated patients, 34 could be transplanted. Immunosuppressive therapy was decided on patient own immunological risk profile rather than HCV infection and or response to treatment. HCV-RNA just before renal transplant and 3–4 months following transplantation did show relapse in 6 patients, thus keeping SVR of 54%.
Discussion
Therapy of HCV is expected to change significantly in time to come and we may look forward to non INF-based therapy even in patients on MHD and renal transplant also. However, until it is applicable, it will be worthwhile to review the current status of INF-based treatment of HCV in MHD patients. The present study is the first single center study from India in sizeable number of patients showing SVR in 54% of the patients with minimal dropout rate. Previously published studies of Peg-INF monotherapy in these patients are shown in Table 3 for comparative analysis.[2425262728293031323334353637383940414243444546474849] The goal of the treatment of HCV-infected patient is to reduce all-cause mortality and liver-related health adverse consequences, including end-stage liver disease and hepatocellular carcinoma, by achieving virological cure as evidenced by an SVR. Although patients treated with either conventional or Peg-IFN-a have similar efficacy and safety results on the basis of meta-analysis studies, one head-to-head randomized trial showed that the overall efficacy and safety in patients treated with Peg-IFN-a were superior to those treated with conventional IFN-α.[36] In published studies with Peg-INF in these patients, SVR had ranged from 0% to 92%. One of the major variables affecting these results has been number of patients included in the studies, which has ranged from 3 to 102. Obviously, results in percentage in studies with small numbers of patients are misleading. If we only include studies with at least 50 patients, there are only five studies including present study with SVR ranging between 14% and 50%.[20424548] There is no study that has compared Peg-INF α2A and Peg-INF α2b in these patients.

Response rate to Peg-IFN in HCV infection also varies as per the genotype of the virus. Though genotype in dialysis unit is primarily affected by the frequency of genotype infection in population, due to nosocomial mode of spread, it is possible that frequency of genotype in patients on dialysis may differ from general population due to accumulation of patients of one particular genotype. In the present study, genotype-1 was present in 41% patients followed by genotype-3. There has been variable frequency of genotype-1 in different studies. While five studies had not mentioned genotype status, in rest of the studies, frequency of genotype-1 has ranged from 20% to 100%. In five studies with sizeable number of patients, genotype-1 had ranged between 41% and 100%. There are seven studies in dialysis patients with all patients having genotype-1 infection but still SVR had been 33–75%. Almost all of these studies had not reported duration of HCV infection before treatment started except one study,[29] where mean duration of infection was 41 months before start of treatment. Viral load in all the studies was between 105 and 107 and, therefore, it was difficult to separate out impact of viral load on response to therapy in these patients. In our study also mean viral load was 106 copies.
SVR in HCV has been reported to be more likely in patients who had taken at least 80% of all scheduled IFN injections for at least 80% of the anticipated duration of treatment.[50] The two most important factors affecting dose and duration are cost of therapy and drug side effects. We have started therapy in patients after explaining cost and duration in great detail. Still, we had 3 of 85 stopping treatment very early because of cost. In previous studies, cost had not been discussed, may be at most places, treatment is supported by medical providers or insurance. In India, most of treatments are self-funded, so cost is an issue for sustaining prolong and costly treatment. In our cohort, there was 5% dropout rate (3 leukopenia and 1 thrombocytopenia). None of the patients had depression or severe asthenia limiting continuation of therapy. In other published studies, dropout rate due to side effects had ranged between nil to 50%. Again, if we take studies with sizeable number of patients,[20424548] treatment-limiting side effects were in 4–32%. Most common reported side effects in these patients were hematological, infection, depression, and asthenia. Although it has been reported that the rates of IFN dose reduction and discontinuation were similar among subjects receiving Peg-IFN and conventional IFN,[51] it is experience of many that tolerability of Peg-IFN is much better and also the convenience of once a week injection. Peg-IFN monotherapy has also been recommended for patients with contraindications to ribavirin, such as those with renal insufficiency, hemoglobinopathies, and ischemic cardiovascular disease.[52] However, recently ribavirin has been used in these patients with modified doses, and many consider ribavirin only a relative contraindication in patients with CKD and dialysis.
In nondialysis patients, RVR has been correlated with SVR in most clinical setting. There are six studies[364344454648] who had reported RVR rate ranging between 32% and 60%. Of these, only two studies[4548] assessed correlation between RVR and SVR and showed a positive correlation. In the present study, RVR was a major predictor of SVR and very few additional patients showed SVR if they did not achieve RVR. In fact, if patient did not respond in 3 months, we started stopping treatment as these patients were unlikely to respond. We had not seen any patient having breakthrough HCV infection while on treatment.
With the availability of direct anti-viral agents (DAA), the scenario of treatment of HCV in dialysis population is also likely to change. Though until now there is no published randomized trial of DAA in dialysis population, there is an ongoing trial of sofosbuvir and ribavirin in hemodialysis population. Our own personal experience suggest that this combination is likely to show a very good response if carefully given in these patients (unpublished data).
Our study has limitation of being a retrospective study. However, due to cost of therapy limiting the enrollment of patients, unless the cost of treatment is supported by funding agency or sponsor, it will be difficult to do a prospective study of patients on hemodialysis HCV infection, more so in Indian context.
Conclusion
Peg-INF monotherapy in patients with HCV infection in Indian population is well-tolerated with reasonable SVR. The response rate was not affected by genotype status. Very few patients required discontinuation of therapy due to side effects and dropout rate had been minimal. RVR was strong predictor of SVR. Until, safety and efficacy of DAA are established in these patients, Peg-INF will remain an acceptable option for treatment before renal transplant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge the support of all the residents of the Department of Nephrology for their contribution in follow-up of these patients.
References
- Hepatitis C virus infection in haemodialysis: The 'no-isolation' policy should not be generalized. Nephron Clin Pract. 2009;111:c133-40.
- [Google Scholar]
- Hepatitis C virus infection during haemodialysis in India. J Assoc Physicians India. 1999;47:1139-43.
- [Google Scholar]
- Hepatitis C virus infection in dialysis and renal transplantation. Kidney Int. 1997;51:981-99.
- [Google Scholar]
- Hepatitis C in a hemodialysis unit: Molecular evidence for nosocomial transmission. J Clin Microbiol. 1998;36:3040-3.
- [Google Scholar]
- Results of renal transplantation on conventional immunosuppression in second decade in India: A single centre experience. J Assoc Physicians India. 2002;50:532-6.
- [Google Scholar]
- Impact of hepatitis C virus infection on renal transplant outcome in India – A single centre study. J Assoc Physicians India. 2000;48:1155-9.
- [Google Scholar]
- HCV infection during renal replacement therapy: Should we dialyze all HCV-positive patients on dedicated machines? Nephron. 1998;79:479-80.
- [Google Scholar]
- Assessment of awareness regarding universal precaution among the nursing staff of AIIMS in 1997. J Assoc Physicians India. 1998;46:1061.
- [Google Scholar]
- Guideline 4: Management of HCV-infected patients before and after kidney transplantation. Kidney Int. 2008;73(Suppl 109):S53-68.
- [Google Scholar]
- Virological and histological responses to one year alpha-interferon-2a in hemodialyzed patients with chronic hepatitis C. Nephron. 2001;88:120-6.
- [Google Scholar]
- Interferon treatment for chronic hepatitis C virus infection in uremic patients. Kidney Int. 1994;45:1507-9.
- [Google Scholar]
- Efficacy and tolerance of alpha-2b interferon therapy on HCV infection of hemodialyzed patients. Kidney Int. 1995;47:1412-8.
- [Google Scholar]
- Interferon-alpha in chronic hepatitis C infection in dialysis patients. Am J Kidney Dis. 1999;34:55-60.
- [Google Scholar]
- Interferon therapy in hemodialysis patients with chronic hepatitis C virus infection induces a high rate of long-term sustained virological and biochemical response. Clin Nephrol. 2001;55:220-6.
- [Google Scholar]
- Evidence that clearance of hepatitis C virus RNA after alpha-interferon therapy in dialysis patients is sustained after renal transplantation. J Am Soc Nephrol. 2003;14:2092-8.
- [Google Scholar]
- The impact of hepatitis C virus viremia on renal graft and patient survival: A 9-year prospective study. Am J Kidney Dis. 2004;43:131-9.
- [Google Scholar]
- The tolerance and efficacy of interferon-alpha in haemodialysis patients with HCV infection: A multicentre, prospective study. Nephrol Dial Transplant. 2001;16:1017-23.
- [Google Scholar]
- Pegylated-interferon alpha 2a treatment for chronic hepatitis C in patients on chronic haemodialysis. World J Gastroenterol. 2006;12:4191-4.
- [Google Scholar]
- Efficacy and tolerability of pegylated-interferon alpha-2a in hemodialysis patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:575-80.
- [Google Scholar]
- Pegylated interferon and ribavirin treatment for hepatitis C in haemodialysis patients. J Viral Hepat. 2006;13:316-21.
- [Google Scholar]
- The treatment of chronic hepatitis C with peginterferon alfa-2a (40 kDa) plus ribavirin in haemodialysed patients awaiting renal transplant. J Hepatol. 2007;46:768-74.
- [Google Scholar]
- Monotherapy with pegylated interferon alpha-2a in hemodialyzed patients with chronic hepatitis C. Dial Transplant. 2008;37:204-108.
- [Google Scholar]
- Prospective randomized control trial of isoniazid chemoprophylaxis during renal replacement therapy. Transpl Infect Dis. 2005;7:99-108.
- [Google Scholar]
- Liver biopsy in patients on hemodialysis with hepatitis C virus infection: An important tool. Indian J Nephrol. 2015;25:152-7.
- [Google Scholar]
- Lupus activation with cerebritis following pegylated interferon in a hemodialysis patient. Nat Rev Nephrol. 2009;5:599-603.
- [Google Scholar]
- Pegylated interferon-alpha 2b monotherapy for haemodialysis patients with chronic hepatitis C. Aliment Pharmacol Ther. 2004;20:123-4.
- [Google Scholar]
- Treatment of hepatitis C virus with interferon in hemodialysis patients awaiting kidney transplant. Transplant Proc. 2005;37:1424-5.
- [Google Scholar]
- Pegylated interferon for the treatment of hepatitis C virus in haemodialysis patients. Nephrol Dial Transplant. 2005;20:991-3.
- [Google Scholar]
- Analysis of safety and efficacy of pegylated-interferon alpha-2a in hepatitis C virus positive hemodialysis patients: Results from a large, multicenter audit. J Nephrol. 2006;19:794-801.
- [Google Scholar]
- Randomized trial of pegylated interferon alpha-2b monotherapy in haemodialysis patients with chronic hepatitis C. Nephrol Dial Transplant. 2006;21:437-43.
- [Google Scholar]
- Preliminary results of treatment with pegylated interferon alpha 2A for chronic hepatitis C virus in kidney transplant candidates on hemodialysis. Transplant Proc. 2007;39:2125-7.
- [Google Scholar]
- Treatment of hepatitis C in hemodialysis patients with pegylated interferon alpha-2a as monotherapy. Ren Fail. 2007;29:961-6.
- [Google Scholar]
- Pilot study of pegylated interferon-alpha 2a in dialysis patients with chronic hepatitis C virus infection. Nephrology (Carlton). 2007;12:11-7.
- [Google Scholar]
- Anemia associated with pegylated interferon-alpha2a and alpha2b therapy in hemodialysis patients. Clin Nephrol. 2007;67:366-73.
- [Google Scholar]
- Monotherapy with peginterferon alpha-2b {12 kDa} for chronic hepatitis C infection in patients undergoing haemodialysis. Trop Gastroenterol. 2007;28:16-8.
- [Google Scholar]
- Pegylated interferon alpha-2a versus standard interferon alpha-2a for treatment-naive dialysis patients with chronic hepatitis C: A randomised study. Gut. 2008;57:525-30.
- [Google Scholar]
- Efficacy and safety of pegylated-interferon alpha-2a in hemodialysis patients with chronic hepatitis C. World J Gastroenterol. 2008;14:255-9.
- [Google Scholar]
- The response to pegylated interferon alpha 2a in haemodialysis patients with hepatitis C virus infection. Infection. 2008;36:341-4.
- [Google Scholar]
- Treatment of hepatitis C in hemodialysis patients using pegylated interferon alpha-2a in Turkey. J Gastroenterol. 2009;44:353-8.
- [Google Scholar]
- Safety and efficacy of an escalating dose regimen of pegylated interferon alpha-2b in the treatment of haemodialysis patients with chronic hepatitis C. J Viral Hepat. 2010;17:410-8.
- [Google Scholar]
- Pegylated interferon for treatment of chronic hepatitis C in hemodialysis patients in Croatia. Kidney Blood Press Res. 2011;34:53-7.
- [Google Scholar]
- Low-dose peginterferon alfa-2a is safe and produces a sustained virologic response in patients with chronic hepatitis C and end-stage renal disease. Clin Gastroenterol Hepatol. 2011;9:242-8.
- [Google Scholar]
- Treatment of hepatitis C virus infection in patients on maintenance hemodialysis: A single United Arab Emirates center experience. Eur J Intern Med. 2011;22:582-6.
- [Google Scholar]
- Treatment of chronic hepatitis C in end stage renal disease: Experience at a tertiary care centre. Trop Gastroenterol. 2012;33:189-92.
- [Google Scholar]
- Pegylated interferon-a2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: A randomized trial. Ann Intern Med. 2013;159:729-38.
- [Google Scholar]
- Efficacy and safety of pegylated interferon alfa-2b and ribavirin combination therapy versus pegylated interferon monotherapy in hemodialysis patients: A comparison of 2 sequentially treated cohorts. Am J Kidney Dis. 2013;62:789-95.
- [Google Scholar]
- Virological responses of pegylated interferon alpha-2a treatment in hemodialysis patients infected with hepatitis C. Clin Exp Nephrol. 2013;17:115-9.
- [Google Scholar]
- Multicenter study of pegylated interferon α-2a monotherapy for hepatitis C virus-infected patients on hemodialysis: REACH study. Ther Apher Dial. 2014;18:603-11.
- [Google Scholar]
- Efficacy and tolerability of low-dose interferon-α in hemodialysis patients with chronic hepatitis C virus infection. World J Gastroenterol. 2014;20:4071-5.
- [Google Scholar]
- Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061-9.
- [Google Scholar]
- Do differences in pegylation of interferon alfa matter? Gastroenterology. 2010;138:34-6.
- [Google Scholar]
- Evolution of interferon-based therapy for chronic hepatitis C. Hepat Res Treat. 2010;2010:140953.
- [Google Scholar]







