Translate this page into:
Comparison of outcomes between surgically placed and percutaneously placed peritoneal dialysis catheters: A retrospective study
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
There is lack of adequate data on comparison of outcomes between percutaneously placed peritoneal dialysis (PD) catheters inserted by nephrologists and PD catheters placed by surgeons. The aim of this study is to retrospectively analyze the outcomes of PD catheters inserted by surgeons (by open surgical or laparoscopic technique) and compare them with those inserted by nephrologists among ESRD patients who underwent elective PD catheter insertions between January 2009 and December 2012. The primary outcome measure was the proportion of catheters removed because of primary nonfunction. The secondary outcome measures were catheter survival, patient survival, and incidence of complications of catheter insertion. A total of 143 PD catheter insertions (88 by surgeons and 55 by nephrologists) performed in 132 patients were considered for the analysis. The primary nonfunction rate of PD catheter insertions in both groups was comparable (18.2% and 7.3%, P = 0.08). Break-in period was shorter in Group N (p = <0.001). No differences were noted in patient or catheter survival. Percutaneously placed PD catheters performed by nephrologists have comparable outcomes with surgically placed PD catheters among selected cases and have the advantage of lower costs, avoidance of operation theater scheduling issues, smaller incision length, and shorter break-in period. Therefore, more nephrologists should acquire the expertise on percutaneous PD catheter placement as it leads to lesser waiting times and better utilization of PD.
Keywords
Catheter malfunction
catheter survival
peritoneal dialysis
seldinger technique
Introduction
Continuous ambulatory peritoneal dialysis (CAPD) is an effective and convenient, home-based modality of renal replacement therapy. The backbone of a successful CAPD program is the success rate of peritoneal dialysis (PD) catheter insertion. PD catheter placement is largely done by the surgeons using the open surgical technique. However, getting a surgeon dedicated to PD catheter insertion is difficult in Indian setting. Moreover, the PD catheter insertion by surgeons is faced with certain issues like requirement of an operation theater and an anesthetist; higher costs and delay in catheter insertion because of scheduling issues in a busy operation theater. These factors eventually lead to a decrease in PD penetration and utilization. However, with the advent of percutaneous method of PD catheter insertion, nephrologists have started placing PD catheters. A multicenter analysis of retrospective data from three centers in USA has shown that PD catheter insertion by nephrologists can lead to improvement in PD utilization.[1] Similar observation was also made by Kelly et al.[2] in his study. Percutaneously placed PD catheters have comparable outcomes as compared to surgically placed catheters as shown by Ozener et al.[3] There are very few studies that have compared the outcomes of PD catheters placed by surgeons vis-a-vis nephrologists in India, with adjustment of confounding factors. Hence, we conducted a retrospective study to analyze the outcomes of PD catheters inserted by the nephrologists (Group N) and the surgeons (Group S) in our institution from January 2009 to December 2012 after adjustment of various confounding factors.
Methods
We studied the outcomes of a retrospective cohort of adult end-stage renal disease (ESRD) patients who underwent elective PD catheter insertion from January 2009 to December 2012 at All India Institute of Medical Sciences which is a Tertiary Care Teaching Hospital and a Research Institute in India. The study was approved by the Institute's Ethics Committee. We excluded the following patients from our study: Patients who had major abdominal surgery before PD catheter placement, patients who had to undergo concomitant abdominal hernia repair or other abdominal surgeries along with PD catheter placement, patients who were critically ill, patients who were morbidly obese (body mass index [BMI] >30) and patients with past history of recurrent PD catheter-related peritonitis, and patients who underwent laparoscopic PD catheter placement.
The primary outcome measure was proportion of PD catheters removed because of primary nonfunction of the catheter in Group N and Group S. Primary nonfunction of PD catheter was defined as catheter malfunction immediately after its insertion or later resulting in inability to perform CAPD exchanges prior to removal of the catheter.
The secondary outcome measures were – catheter survival, patient survival, catheter infection rate (exit site infection or tunnel infection), peritonitis rate, and incidence of the peri-catheter leak.
Data collection
We reviewed the departmental CAPD audit data to gather information on all elective PD catheter insertions done from January 2009 to December 2012. The information obtained from the departmental CAPD registry included the following: the date of catheter insertion, indication for CAPD, type of catheter used, inserting surgeon/physician, and catheter position after its insertion, status of catheter function at first flushing, and any mechanical or nonmechanical complications after its insertion. We then retrieved the in-patient records of those patients who were found eligible for the study. Information regarding co-morbidities, the presence of any additional exclusion criteria and the immediate postop course were obtained from these inpatient records. The details regarding number of peritonitis episodes after discharge, date of catheter removal and its reasons, survival status of the catheter, and the patient as on July 07, 2013 were obtained from outpatient follow-up records, PD nurses and by telephonic enquiry from the patients themselves. In case a patient underwent PD catheter insertion more than once, then each catheter insertion was considered a separate event.
Catheter placement procedure
In Group S, PD catheters were predominantly placed by two surgeons (one surgical consultant assisted by a resident) using the open surgical technique. All catheters were placed by the same surgical consultant, but the residents who assisted in catheter placement varied depending on rotation. The surgeons placed the catheter after a mini-laparotomy under short general anesthesia or local anesthesia with mild sedation in the main operation theater. The surgical insertion briefly consisted of the following steps: a vertical left paramedian incision was given 2–3 cm lateral to the midline, the abdominal layers were dissected and the parietal peritoneum opened by a small knick just enough for the catheter to pass through it. The catheter was then introduced through the peritoneal opening, directed toward the left pelvis with the help of a stylet. The internal cuff was placed in the preperitoneal space and was then sutured with the parietal peritoneum. The anterior rectus sheath was then closed, and the catheter was brought out through a subcutaneous tunnel with the external cuff lying within the tunnel and approximately 2 cm from the exit site. The direction of the tunnel was usually directed caudolaterally.
In Group N, the PD catheters were inserted by nephrologists using the Seldinger technique[4] with the help of a catheter insertion kit (Quinton) under local anesthesia. Initially, the insertions were performed by two consultant nephrologists trained in percutaneous PD catheter insertion assisted by residents. Subsequently, three more nephrology residents were trained by the consultant nephrologist to do the procedure. The residents trained in inserting the catheters always inserted the catheters under the supervision of the senior nephrologist. The main steps of catheter insertion by nephrologist consisted of following: left vertical paramedian incision was given just medial to the lateral border of the rectus muscle. The soft tissues were dissected up to the anterior rectus sheath. After opening the anterior sheath, the posterior sheath was visualized by separating the muscle fibers with a curved artery forceps. After visualization of the posterior sheath, a 16 gauge introducer needle was used to prick the posterior sheath until the tip entered the peritoneum after which a guidewire was advanced through the needle into the peritoneum. Subsequently, a peel-away sheath with an introducer was advanced over the guidewire. The guidewire and introducer were then removed. The Tenckhoff double cuff CAPD catheter was then introduced through the peel-away sheath with the help of a stylet directed toward the left iliac fossa. Once the inner cuff of the catheter reaches the plane of the posterior sheath, the stylet was removed. The peel away sheath was subsequently peeled off, and the internal cuff was secured between the muscles and sutured with anterior rectus sheath. After checking inflow and outflow through the catheter by instilling 500 ml of 2.5% PD fluid, a subcutaneous tunnel directed caudolaterally of about 8–12 cm length was made using the tunneller and planned in such a way that the external cuff was placed in the tunnel 2 cm from the exit site. Before taking the proximal portion of the catheter through the tunnel, a sling was made in order to maintain the curvature of the catheter in the subcutaneous tissue. The main differences in the steps of PD catheter insertion including pre- and post-operative management between surgeons and nephrologists (Group S and Group N) are briefly summarized in Table 1.

Statistical analysis
The means of continuous variables were compared across the groups using the Student's t-test for independent samples. Categorical variables across the groups were compared using Chi-square test. The cumulative probability of patient survival and catheter survival at 1-year, 2 years, and on July 31, 2013 was computed by the Kaplan–Meier technique. The catheter survival was calculated based on the status of the catheter (functional or removed) as on July 31, 2103 after censoring for the following events: switch to hemodialysis (HD) by preference, transplantation, death, loss to follow-up, and recovery of native renal function. We used the Statistical Package for the Social Sciences (SPSS) Software package (version 18.0, SPSS, Chicago, IL, USA) for statistical analyses. A P = 0.05 was considered as statistically significant.
Results
Overall, 155 PD catheter insertions were performed during the study period. After excluding 12 PD catheter insertions based on the exclusion criteria, the remaining 143 PD catheter insertions performed in 132 patients were considered for the final analysis. The reason for exclusion of the 12 cases was as follows: five patients underwent concomitant abdominal surgery; two patients were morbidly obese with BMI of more than 30 at the time of catheter insertion; two patients had history of major abdominal surgery prior to PD catheter placement; one patient underwent laparoscopic PD catheter placement; two patients were excluded because their follow-up records could not be traced. Of these 143 PD catheter insertions, 88 were done by the surgeons using the open surgical technique (Group S) while 55 PD insertions were performed by the nephrologists (Group N). In the initial part of the study period, there were two trained nephrologists who used to place PD catheters and, therefore, the frequency of catheter placement was subject to the availability of the trained nephrologists. Subsequently, three more nephrologists were also trained to perform the PD catheter insertion by percutaneous technique. Hence, increasing proportions of catheter placements were done by the nephrologists toward the end of the study period. Figure 1 shows the proportion of the PD catheters inserted by nephrologists and surgeons year wise. The baseline characteristics of the patients belonging to Group S and Group N were comparable [Table 2].

- The number of peritoneal dialysis catheter insertions done by surgeons and nephrologists year wise from 2009 to 2012

Primary outcome measure
The primary nonfunction rate of PD catheter insertions in Group S (16/88 [18.2%]) was numerically higher than Group N (4/55 [7.3%]) but did not reach statistical significance (P = 0.08) [Table 3]. Most of the cases of primary catheter nonfunction in both the groups were attributed to malposition of the catheter tip leading to outflow failure (15 in Group S and two in Group N) and the remaining cases (two in Group S and two in Group N) had omental wrapping confirmed on PD catheterography. About 50% of patients who developed primary catheter failure underwent PD catheter re-insertion in both the groups (2 of 4 in Group N and 9 of 17 in Group S). In all cases of primary failure, the subsequent PD catheter placement was done using the same technique, and there was no cross-over of patients in both groups.

Secondary outcome measures
The secondary outcome measures in both the groups are shown in Table 3. The cumulative probabilities of catheter as well as patient survival were comparable [Figures 2 and 3]. There were no differences in peritonitis rate, exit site infection rate, exit site bleeding or peri-catheter leak across both the groups. None of the patients in either group experienced tunnel infection, visceral injury, or wound hematoma post catheter insertion.

- Kaplan–Meir curves for catheter survival in Group S (continuous ambulatory peritoneal dialysis catheter inserted by surgeons) and Group N (catheter inserted by nephrologists)
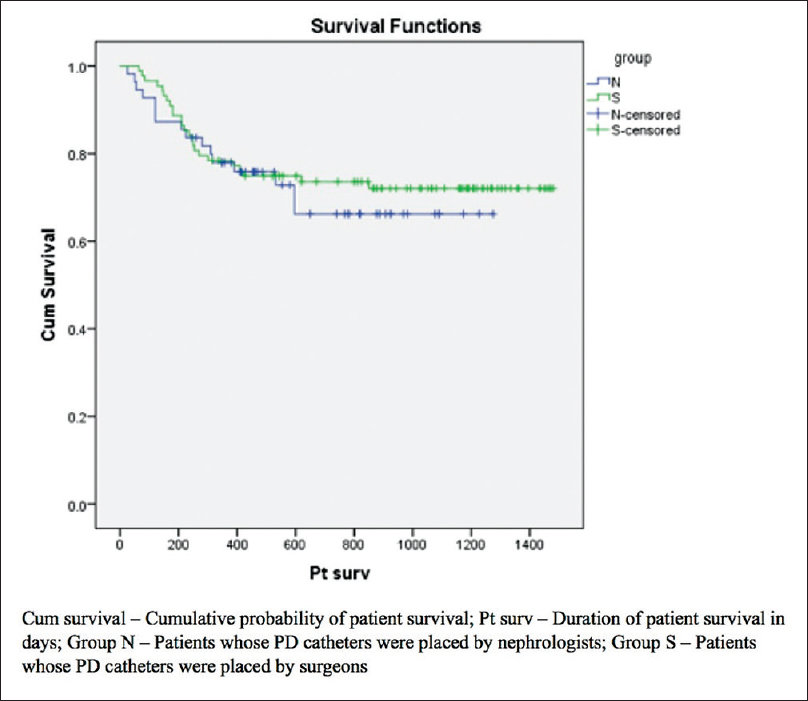
- Kaplan–Meir curves for patient survival in Group S (continuous ambulatory peritoneal dialysis catheter inserted by surgeons) and Group N (catheter inserted by nephrologists)
The average break-in period (duration between insertion of PD catheter and initiation of regular exchanges) was significantly shorter in Group N (9.7 ± 0.8 vs 13.9 ± 3.9 days, P < 0.001). The most common indications for CAPD initiation in both the groups were: patient's choice (mainly because of poor access to HD center) and multiple vascular access failures leading to transfer from HD.
Overall, 54 patients in the Group S (61%) and 34 patients in Group N (62%) underwent catheter removal during the study period. The reasons for catheter removal in both groups are summarized in Table 3. Catheter malfunction (37%) followed by death of the patient (31.4%) were the two most common causes of catheter removal in Group S. In Group N, death of the patient (35.2%) was the most common cause of catheter removal followed by catheter malfunction and refractory peritonitis (17.6% each).
Discussion
This study shows that the rate of primary catheter nonfunction among PD catheters inserted by dedicated and trained nephrologists was comparable with that of surgically placed PD catheters. In addition, the break-in period was shorter in catheters placed by nephrologists as compared to surgically placed catheters without an increase in the rate of complications. The Seldinger technique was chosen as the method of choice by the nephrologists mainly because it could be performed in a procedure room of a dialysis unit without requirement of an operation theater or general anesthesia and the procedure could be performed on any date without any scheduling issues. The progressive increase in the percentage of PD catheter placements by nephrologists from 5% in 2009 to 25% in 2012 in our center demonstrates the simple learning curve for the PD catheter insertion procedure. The comparable rates of primary catheter nonfunction in Group N (7.3%) as compared to Group S (18.2%) (P = 0.08) is in agreement with the previous studies.[3] The tip of the PD catheter was not positioned appropriately in the true pelvis in about 19.3% of the surgically inserted catheters and 10.9% of percutaneously inserted catheters. The slightly higher rate of improper positioning of PD catheter tip is Group S might be responsible for the slightly higher (although statistically nonsignificant) rate of primary catheter failure in that group. Crabtree[5] had pointed out in his study that the low rate of primary failure in the percutaneously placed catheters reported by some studies might have occurred because of selection bias wherein the surgeons had to insert PD catheters in complicated cases. To avoid this selection bias, we excluded insertions performed in complicated cases (as mentioned above) from our study. In order to ensure the maintenance of the curvature of the intramural segments and to overcome the problem of silastic resistance or shape-memory of straight Tenckhoff catheters, the nephrologists at our institute created a sling in the subcutaneous plane using 3–0 prolene [Figure 4]. This sling might have partly contributed to the relatively lower incidence of early catheter migration and hence primary catheter failure in the Group N. However, the role of such a sling in preventing catheter migration needs to be studied in trials with prospective controlled design. Tahawar et al.[6] studied the outcomes of medically and surgically placed PD catheters in a university teaching hospital at Plymouth wherein they reported a slightly higher (but statistically insignificant) rate of primary failure in medical (16.7%) than surgical PD catheter insertions. However, in that study, percutaneously placed catheters were placed by midline approach and surgically placed catheters were placed by paramedian approach, unlike our study in which the nephrologist used the paramedian approach. These factors might have influenced the outcomes observed in both the studies. In addition, the study done by Tahawar et al. did not adjust for the catheter design and presence of complicating factors like previous surgeries and number of re-insertions and therefore there were more number of complicated cases in surgical group than medical group making the comparisons difficult. The break-in period was significantly shorter in Group N than in Group S with no significant difference in the incidence of pericatheter leak. This is an added advantage of the percutaneous technique where regular CAPD exchanges can be started much early compared to surgical insertion. Some studies have reported higher rates of early pericatheter leaks with the percutaneous method.[7] This is probably because in many centers, the percutaneous method of PD catheter insertion is done using the midline approach and the cuff is therefore not placed in the muscular plane. However, the nephrologists in our institute used a modification of seldinger technique wherein the deeper cuff was advanced to the muscular plane after opening the anterior rectus sheath as suggested by the guidelines.[8] This might have resulted in much lower incidence of pericatheter leaks despite a short break-in period. The rate of peritonitis, exit site and tunnel infections, and other mechanical complications were comparable between the two groups. Kaplan–Meir analysis of the cumulative probability of catheter and patient survival at 12 months, 24 months and at completion of study showed an increase in number of early catheter loss in the surgical group. However, there was no significant difference in catheter and patient survival at the completion of the study. Ozener et al.[3] did a large retrospective study comparing the performance of percutaneously placed PD catheters with surgically placed PD catheters and observed a better survival of percutaneously placed PD catheters. However, in that study, all the patients who underwent surgical insertion of PD catheters were historical controls who had underwent catheter insertion prior to 1996 and the type of catheter also differed significantly between the two groups. These factors might have played a role in differences in outcomes between the two groups of patients. However, the catheter survival and complications rates were similar in both medical and surgical groups as observed in our study. Sampathkumar et al.[9] have published 4 years data of PD catheter insertions performed by surgeons and nephrologists. However, the study had certain limitations – a number of patients in surgical group was small (19 patients), rate of primary catheter malfunction was not mentioned in the study, baseline characteristics of both groups were not presented and there was no mention on type of catheter used. Besides, the study also reported a higher rate of pericatheter leak (4%) probably because of very early initiation (break-in period of 4 days). Complication rates were slightly higher in that study including-eight cases of surgical conversion mainly for re-positioning, four cases of bowel injury and one case of bladder injury. There was no mention on whether the all the cases were performed by the same nephrologists and whether catheter insertions performed by less experienced nephrologists were supervised or not as this may have great impact on outcomes.

- Schematic illustration of how a subcutaneous sling is placed around the inter-cuff segment of the catheter to maintain its curvature and prevent it from straightening
It is to be noted that the nephrologists at our institute did not use ultrasound and fluoroscopic guidance to penetrate the peritoneum and position the PD catheter respectively due to nonavailability of these facilities in the department. In a study by Jo et al.,[10] 51 PD catheters were inserted by the Seldinger technique without ultrasound or fluoroscopic guidance and only one catheter had primary malfunction (primary malfunction rate of 2%). Hence, when placed by well-trained nephrologists, primary malfunction rate of PD catheter after its insertion does not appear to depend much on whether fluoroscopic guidance was used or not. However, it is always a good idea to insert PD catheter using fluoroscopic guidance as it enhances safety and accuracy of the procedure. The needle that was used to puncture the peritoneum was a sharp needle provided along with the kit and there is no clear-cut recommendation on the type of needle to be used to puncture the peritoneum.
However, it is to be noted that these results are applicable to uncomplicated elective PD catheter insertions. There is still a role of surgical PD catheter placement in complicated cases such as – PD catheter placement in patients who have undergone previous abdominal surgeries, patients requiring concomitant hernia repair along with PD catheter placement, patients who are morbidly obese etc.
The strengths of our study are that the comparison of outcomes in both the groups were made after adjustment for factors such as catheter design, co-morbidities and presence of complicating factors that influences the catheter and patient outcomes. The limitations of the study are the retrospective study design, variations in the duration of follow-ups between the two groups and the difference in the technique of catheter insertion in both the groups. But the overall message obtained after analysis of our study and other similar studies was that the outcomes of catheter placement depend more on the skill and experience of the inserting surgeon rather than the actual technique. So each center should study the outcomes of the catheter insertion (in otherwise uncomplicated cases) done by surgeons and nephrologists and depending on the outcome should decide who should insert PD catheter. A prospective randomized controlled study comparing the outcomes of PD catheter placed by surgeons and nephrologist would further clarify the unresolved issues. Whether the creation of sling in the subcutaneous space prevents, catheter migration also needs to be studied in a randomized fashion.
Conclusion
Percutaneously placed PD catheters performed by a dedicated and well-trained nephrologists using a paramedian approach is associated with comparable outcomes with a shorter break-in period as compared to PD catheters inserted by surgeons in otherwise uncomplicated ESRD patients undergoing elective PD catheter insertion.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Does catheter insertion by nephrologists improve peritoneal dialysis utilization? A multicenter analysis. Semin Dial. 2005;18:157-60.
- [Google Scholar]
- Peritoneoscopic peritoneal dialysis catheter insertion. Nephrology (Carlton). 2003;8:315-7.
- [Google Scholar]
- Technical survival of CAPD catheters: comparison between percutaneous and conventional surgical placement techniques. Nephrol Dial Transplant. 2001;16:1893-9.
- [Google Scholar]
- Fluoroscopic placement of peritoneal dialysis catheters: a harvest of the low-hanging fruits. Perit Dial Int. 2008;28:134-7.
- [Google Scholar]
- Evaluation of medical insertion of peritoneal dialysis catheters, evaluation of medical insertion of peritoneal dialysis catheters. Nephrourol Mon. 2011;03:46-53.
- [Google Scholar]
- Routine percutaneous insertion of permanent peritoneal dialysis catheters on the nephrology ward. Perit Dial Int. 1994;14:284-6.
- [Google Scholar]
- Peritoneal catheters and exit-site practices toward optimum peritoneal access: 1998 update. (Official Report from the International Society for Peritoneal Dialysis) Perit Dial Int. 1998;18:11-33.
- [Google Scholar]
- Percutaneous CAPD cather insertion by nephrologist – A single centre experience over 4 years. Indian J Perit Dial. 2010;18:15-8.
- [Google Scholar]
- Immediate initiation of CAPD following percutaneous catheter placement without break-in procedure. Perit Dial Int. 2007;27:179-83.
- [Google Scholar]







