Translate this page into:
Prevalence of obesity and risk of chronic kidney disease among young adults in Egypt
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Increasing body mass index (BMI) has reached epidemic proportions globally and recently emerged as strong, independent risk factors for chronic kidney disease (CKD). We conducted this study to verify the prevalence of obesity and the associated risk of developing CKD among 3000 Egyptian students. The World Health Organization classification of BMI categorized study population into 1–5 groups, 1146 subjects with normal BMI (20–25), 951 subjects with BMI 25–29.9, 540 subjects with BMI 30–34.9, 225 with BMI 35–39.9, and 138 with BMI above 40. The participants were subjected to clinical examination, anthropometric measurements, laboratory investigation, including urinary albumin/creatinine ratio (ACR) and estimation of glomerular filtration rate (eGFR). The prevalence of overweight, obesity, and metabolic syndrome (MS) was 31.7%, 30.1%, and 16%, respectively. The prevalence of CKD among subjects with BMI >25 was 6.5%, almost all of them had BMI >35. ACR and eGFR rose progressively with increasing BMI. Elevated mean arterial pressure (MAP), high sensitivity C-reactive protein, and MS increased the risk of development of CKD. Moreover, MAP, waist to height ratio, and triglycerides to high-density lipoprotein ratios at levels of >95 mm Hg, >0.6, and >3 had sensitivity 91.7%, 88.4%, and 86.7%; and specificity 92.3%, 96.4%, and 96.5%, respectively to predict CKD. The prevalence of obesity among Egyptian young adults was high (30.1%) and was associated with increased the risk of CKD (6.5%).
Keywords
Body mass index
chronic kidney disease
metabolic syndrome
urinary albumin/creatinine ratio
Introduction
Obesity has become a global epidemic.[1] Metabolic syndrome (MS), a major consequence of obesity, is also on the rise. The components of MS include central obesity, elevated blood pressure (BP), impaired fasting glucose, high triglycerides (TG), and low high-density lipoprotein (HDL) cholesterol levels, and these components are present in approximately 20% adults in the USA.[2] It has also been suggested that the plasma TG/HDL ratio can serve as a simple and easily accessible marker for the diagnosis of MS and insulin resistance.[3]
Chronic inflammation is another feature of the MS, which, together with insulin resistance, results in complex metabolic derangements that contribute to the pathogenesis of hypertension, lipoprotein abnormalities, atherosclerosis, coronary artery disease, and chronic kidney disease (CKD).[4]
CKD is now one of the major public health problems. Early detection of CKD is crucial to prevent its progression, and thereby, to potentially improve its outcome.[56]
The histopathology of proteinuric obese patients consists of glomerulomegaly with or without focal segmental glomerulosclerosis (FSGS). These patients tend to have less podocyte injury and a more indolent progression than patients with idiopathic FSGS. These glomerular changes have been termed obesity-related glomerulopathy. The latter is thought to be related to altered renal hemodynamics, namely increased renal blood flow, hyperfiltration, and increased filtration fraction.[7]
Although several studies have demonstrated an association between obesity and incident CKD, these observations have been made primarily in cohorts of older individuals.[8] We conducted this study to verify the prevalence of obesity and its associated risk of developing CKD among Egyptian young adults
Methods
Study design and population
A Cross-sectional study was carried out among students of the Zagazig University between January 2014 and April 2015. The study was approved by the Institutional Ethics Committee and conformed to the Helsinki Declaration. The aim of the study was explained, and informed consent obtained from all subjects.
The enrolled number was 3000 participants, among them 1380 males and 1620 females, with mean age of 22.8 ± 2.6, ranged from 18 to 25 years.
The participants were excluded from the study if they gave a history of renal diseases, hypertension, diabetes mellitus, or other chronic diseases. Adults with a body mass index (BMI) <20 were also excluded.
According to the World Health Organization classification of BMI,[9] the study populations were categorized into five groups: 1146 subjects with normal BMI (20–25); 951 over weight subjects (BMI 25–29.9); 540 with grade I obesity (BMI 30–34.9); 225 with grade II obesity (BMI 35–39.9) and 138 having morbid obesity (BMI >40).
MS was defined by the presence of at least 3 of the following: HDL <40 mg/dl for men or 50 mg/dl for women, fasting blood sugar (FBS) >100 mg/dl, fasting TG level over 150 mg/dl, BP ≥130/85 mmHg, waist circumference ≥102 cm for men or ≥88 cm for women.[2] A total of 479 young adults satisfied the definition of MS.
Physical examination and measurements
All participants were subjected to complete clinical examination and the following anthropometric measurements: height (measured using calibrated height meters while subjects stood erect and barefooted, with feet, placing together, and looking forward), weight (the scale was calibrated daily), BMI (BW/H2 kg/m), WC (the circumference below the costal margin at the level of the umbilicus), and WC to height ratio (WC/H).
Laboratory investigations
All participants were subjected to routine investigations, including FBS, serum creatinine (SCr), high sensitivity C-reactive protein (HS-CRP), lipid profile (HDL, low-Density Lipoprotein (LDL), total cholesterol, and TG. LDL level was calculated according to the Friedewald equation.[10]
Urine samples were examined for urinary albumin/creatinine ratio (ACR), and abnormal albuminuria was defined as a urine ACR ≥30 mg/g.[5] All positive cases re-examined after 3 months to confirm diagnosis.
Choosing the best method to accurately estimate GFR in the obese is not straightforward because of conflicting studies.[11] Ultimately, using the Cockcroft – Gault equation with an adjustment body weight (ABW) seems to be the best measure of renal function in obesity.[1213] Hyperfiltration was defined as a GFR of between 125 and 140 mL/min/1.73 m2.[14]
Statistical analyses
Results were expressed as mean ± standard deviation (SD), analysis of variance by ANOVA and post hoc analysis with LSD tests were applied for comparing differences among groups. Data of HS-CRP and ACR were expressed as median because of skewed distribution and analyzed by Kruskal–Wallis test. Qualitative data were expressed in the form of numbers and percentages and comparison between data was performed by using the Chi-square test. Z-test (test of proportion) was used for comparison of two proportions. The correlation between variables was calculated using the Pearson's and the Spearman correlation tests. Predictive values were assessed by the area under curve/the receiver operator characteristic curve (AUC/ROC). The AUC/ROC was used to determine the discriminatory ability of risk factor in detecting CKD. The criterion for statistical significance was set at P < 0.05. All calculations were carried out using a standard statistical package (SPSS version 19, Inc. in Chicago, USA).
Results
Out of 3000 participants, with the mean age of 22.4 ± 2.8 years, there were 1380 males. BMI categorized the current study into five groups (G1-5). G1 with a normal BMI group represented by 1146 (38.2%), G2 overweight represented by 951 (31.7%), G3 grade I obesity represented by 540 (18%), G4 grade II obesity represented by 225 (7.5%), and G5 grade III obesity represented by 138 (4.6%). Hence, the prevalence of overweight and obesity among Egyptian young adults were 31.7% and 30.1%, respectively.
Among the overweight population, there were 561 (59%) males. The sex difference was significant, (χ2 = 20.6; P < 0.001). In the contrary, the prevalence of obesity was higher in females: Gr I 66%, Gr II 69% and Gr III 74% than the matching level among males, 34%, 31% and 26% respectively (χ2 = 35.2, 22.8 and 21.6; P < 0.001 for all), respectively.
Central obesity was seen in 581 (19.3%) young adults and 31.3% of overweight and obese individuals. The prevalence of central obesity among females was 393 (24.2%), significantly higher than males: 188 (13.6%) (χ2 = 48.9; P < 0.001).
The prevalence of the MS was 479 (16%), which related to BMI (P < 0.001). The prevalence of MS among females was 333 (20.5%), significantly higher than males 146 (10.5%) (χ2 = 49. 5; P < 0.001).
BP, FBS, and HS-CRP were significantly higher in obese than overweight and normal weight young adults and progressively rose with increasing BMI. All obesity indices rose with increasing BMI. Similar all lipid profiles, including TG/HDL, were higher in obese than overweight and normal weight young adults.
SCr levels were normal without significant difference between all groups. Although eGFR was within normal range in all groups, it progressively increased with increasing BMI.
ACR was significantly higher in obese than overweight and normal body weight participants and rose progressively with increasing BMI [Table 1].
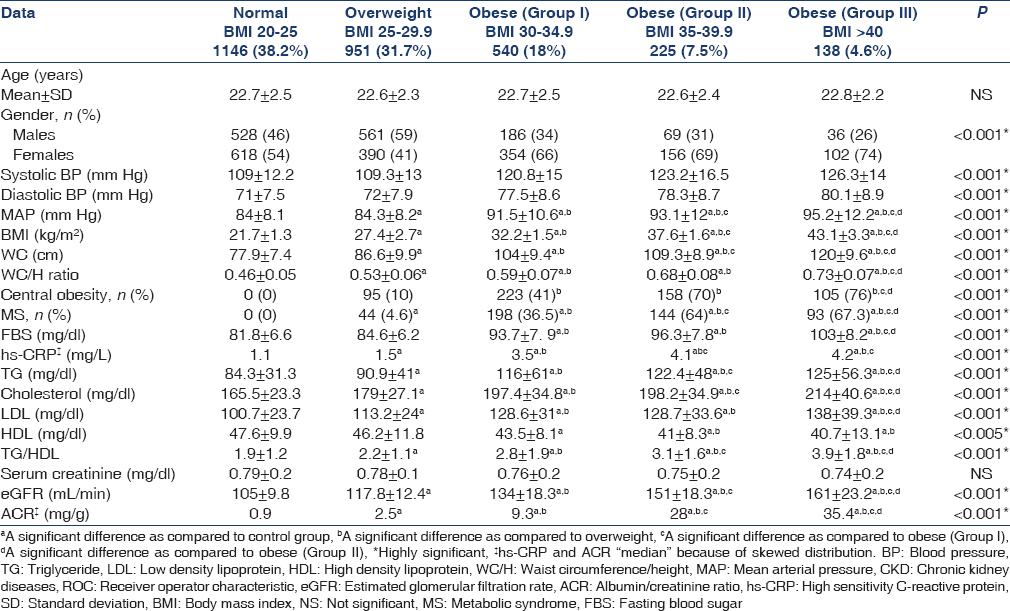
The prevalence of CKD among young adults with BMI >25 was 121 (6.5%), and almost all of them had BMI >35. In addition, about 87 (72%) fulfilled two or more components of MS. Subjects with MS had a 2.5-fold increased prevalence of CKD than those without MS (χ2 = 16.1; P < 0.001).
FBS, mean arterial pressure (MAP), WC, WC/H ratio, cholesterol, TG, LDL, TG/HDL, HS-CRP, eGFR and ACR were positively correlated with increasing BMI, while HDL negatively correlated with increasing BMI [Table 2].
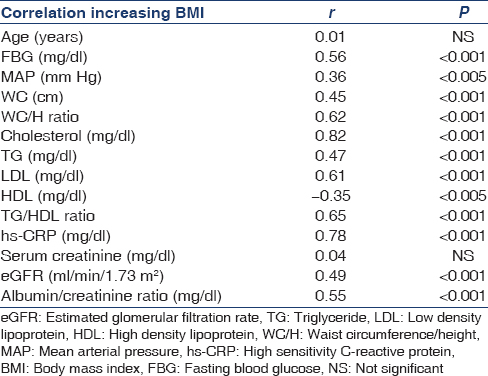
Participants with elevated MAP, WC/H ratio, TG/HDL ratio, HS-CRP, and MS were more likley to have CKD [Table 3].
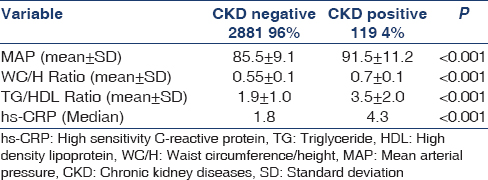
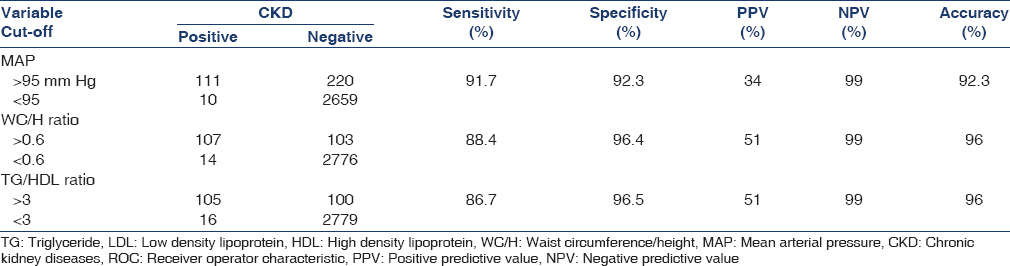
MAP, WC/H, and TG/HDL ratios at levels of >95 mm Hg, >0.6, and >3, respectively, had sensitivity 91.7%, 88.4%, and 86.7%; and specificity 92.3%, 96.4%, and 96.5%, respectively to predict CKD [Table 4].
Discussion
Obesity is becoming a global epidemic in young adults, and is often associated with the presence of the MS and numerous co-morbidities such as cardiovascular diseases, type 2 diabetes, hypertension, certain cancers, and sleep apnea sleep syndrome. Less attention has been paid to the link between increased BMI and CKD, although there is evidence that the steady rise in CKD prevalence may be closely associated with increasing BMI.[15]
The prevalence of overweight and obesity among young Egyptian adults was 31.7% and 30.1%, respectively. Moreover, 18% were grade I obesity, 7.5% grade II, and 4.6% grade III or morbid obesity, besides female gender more obese than males. The current data, highlight the magnitude of the growing problem of obesity among young Egyptian adults. Comparable prevalence of overweight and obesity in adult's ≥20 years was recorded in regional Middle East areas (Libya 30%, 43.7%; Jordan, 37%, 36.5%; Lebanon 38.9%, 27.8%; Qatar 27.8%, 49.3%; Palestine 37.4%, 36.1%; Saudi Arabia 34.4%, 37.2%) respectively.[16] In addition, 33.1% and 35.7% of USA adults are overweight and obese as well 6.3% have extreme obesity.[17]
According to Global Health Observatory data, the prevalence of raising BMI increases with the income level of countries. Although Egypt one of the lower-middle income country, the prevalence of obesity in Egypt is exceeding corresponding countries (8%) and matching upper middle-income levels countries (30%). It may be attributed to poor dietary habit, change in the composition of the diet, increasing use of “junk foods,” changes in the gut microbiome, and lack of physical activity among young Egyptian adults.[18]
The prevalence of MS was high among young Egyptians. Worldwide prevalence estimates for the MS in men range from 8% in India to 24% in the USA and for women from 7% in France to 46% in India.[19]
The health effects of overweight and obesity have been extensively debated. However, the findings of large pooling studies used for the Global Burden of Disease 2013 show consistent risks as BMI reached more than 23 kg/m², especially for cardiovascular disease, cancer, diabetes, osteoarthritis, and CKD.[20]
In the current study, we reported that ACR as an early marker of CKD was significantly higher in obese Gr III and Gr II than obese Gr I overweight and normal body weight participants and progressively uprising with increasing BMI. The prevalence of early CKD among overweight and obese young adults was 6.5% and almost all of them with BMI >35, beside most of them were fulfilled two or more components of MS. MS had a 2.5-fold increased prevalence of CKD than those without MS. Our findings were consistent with other reports that link higher BMI with albuminuria, CKD,[21] and higher prevalence of CKD among MS subjects.[22] Moreover, we found that high TG/HDL ratio, which serve as a simple and easily accessible marker for the diagnosis of MS and insulin resistance,[3] were significantly higher among those with CKD. These data support the importance of MS in the development of CKD. TG/HDL ratio at the level of >3 had high sensitivity and specificity and to predict CKD. The association between TG/HDL and CKD has been reported in another study.[23]
WC/H is an alternative anthropometric index of central obesity and even more appropriate than BMI or WC in predicting CVD risks.[24] In addition, we observed that WC/H ratio >0.6 had high sensitivity and specificity to predict CKD.
MAP reflects the steady-state component of BP and is recognized as an important parameter in clinical nephrology practice.[25] Our findings of MAP have a clinical importance with value >95 mm Hg having high sensitivity and specificity to predict CKD among obese young adults and appeared in agreement with a previous study reporting the optimal target MAP of 90-95 mmHg.[25]
In the current study, we reported that HS-CRP, a marker of low-grade inflammation, progressively increases with rising BMI. Increased fat mass is associated with increased endogenous production of pro-inflammatory cytokines by adipocytes including tumor necrosis factor alpha, CRP, and interleukin-6.[26] CRP is a marker of albuminuria-induced renal injury and a risk marker for renal function loss.[27]
Chronic inflammation is another feature of the MS, which together with insulin resistance, results in complex metabolic derangements that contribute to the pathogenesis of hypertension, lipoprotein abnormalities, high TG/HDL ratio, and all are equally important in pathogenesis of CKD.[4] The mechanisms underlying the contribution of lipids to renal injury are not completely understood. Dyslipidemia is associated with glomerular capillary endothelial and mesangial cell as well as podocyte injury, which further leads to mesangial sclerosis.[28]
Although SCr remained normal in all study groups, progressively rose with increasing BMI indicating increase renal perfusion and hyperfiltration. Early inflammatory processes related to high body fat may predispose the kidney to glomerular hyperfiltration.[29] Visceral obesity and associated compression of the kidneys may increase loop of Henle sodium chloride reabsorption, reducing sodium chloride delivery to the macula densa and causing, via tubule glomerular feedback, reductions in afferent arteriolar resistance and increases in renal blood flow, GFR, and renin secretion.[30] Indeed, several adipocytokines including resistin, adiponectin, and hyperleptinaemia were associated with glomerular hyperfiltration.[31] The association between hypertension and the increases odds of glomerular hyperfiltration, however modest, is not surprising as elevated BP is recognized as a driver of increases in glomerular capillary hydraulic pressure and glomerular filtration.[32] Furthermore, high metabolic risk is associated with glomerular hyperfiltration and augments the risk of microalbuminuria.[33] In the current study, we also showed the association of the inflammatory state, visceral obesity, elevated MAP, and MS were associated early manifestation of CKD.
The glomerular hyperfiltration that occurs after weight gain eventually subsides and may be replaced by a gradual decrease in GFR as renal injury and nephron loss occur in association with prolonged obesity.[34] Such scenario of increasing GFR followed by gradual decline may explain the high GFR in the current study as all participating were young adults with the relatively recent onset of obesity. Moreover, another study observed decline in GFR in later life.[35] Such observation necessitates further work up to follow up GFR in all study populations and also necessitate weight reduction programs to minimize the risk of the gradual decline of renal functions.
Conclusion
Overall, the prevalence of obesity among Egyptian young adults was high (30.1%) and increasing BMI was associated with a higher risk of hypertension, diabetes, inflammatory state, disturbed lipid profile, high TG/HDL, WC/H ratios, MS, increasing ACR, glomerular hyperfiltration, and hence CKD. The prevalence of CKD among obese young adults was 6.5%. Participants with elevated MAP, WC/H ratio, TG/HDL ratio, HS-CRP, and MS were more liable to CKD. Moreover MAP, WC/H, and TG/HDL ratios at levels of >95 mm Hg, >0.6, and > 3 may be useful in clinical practice to predict CKD. Clinicians should monitor obese young adults, especially those with MBI above 35 for evidence of early CKD and advice for weight loss in early adulthood that may have a considerable effect on the development of CKD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-52.
- [Google Scholar]
- Evidence for an independent relationship between insulin resistance and fasting plasma HDL, TG and insulin concentrations. J Am Soc Nephrol. 2004;15:2775-91.
- [Google Scholar]
- The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004;15:2792-800.
- [Google Scholar]
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of CKD. Kidney Int. 2013;3:1-150.
- [Google Scholar]
- Variability and risk factors for kidney disease progression and death following attainment of stage 4 CKD in a referred cohort. Am J Kidney Dis. 2008;52:661-71.
- [Google Scholar]
- Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001;12:1211-17.
- [Google Scholar]
- Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871-80.
- [Google Scholar]
- 2012. World Health Organization. Obesity and overweight. Geneva: World Health Organization; Available from: http://www.who.int/mediacentre/factsheets/fs311/en
- Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- [Google Scholar]
- Interpreting different measures of glomerular filtration rate in obesity and weight loss: Pitfalls for the clinician. Int J Obes (Lond). 2012;36:1421-7.
- [Google Scholar]
- Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247:F632-6.
- [Google Scholar]
- Urinary albumin excretion rate and glomerular filtration rate in the prediction of diabetic nephropathy; a long-term follow-up study of childhood onset type-1 diabetic patients. Nephrol Dial Transplant. 2001;16:1382-6.
- [Google Scholar]
- Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther. 2004;11:41-54.
- [Google Scholar]
- Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-81.
- [Google Scholar]
- Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491-7.
- [Google Scholar]
- Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr Rev. 2004;62:S98-104.
- [Google Scholar]
- The metabolic syndrome: Prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351-75.
- [Google Scholar]
- Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083-96.
- [Google Scholar]
- Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844-50.
- [Google Scholar]
- Metabolic syndrome and chronic kidney disease in an adult Korean population: Results from the Korean National Health Screening. PLoS One. 2014;9:e93795.
- [Google Scholar]
- Waist-to-height ratio, waist circumference, and body mass index as indices of cardiometabolic risk among 36,642 Taiwanese adults. Eur J Nutr. 2013;52:57-65.
- [Google Scholar]
- Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: Clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027-37.
- [Google Scholar]
- Importance of blood pressure control in chronic kidney disease. J Am Soc Nephrol. 2006;17 4 Suppl 2:S98-103.
- [Google Scholar]
- Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745-51.
- [Google Scholar]
- C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63:654-61.
- [Google Scholar]
- Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl. 2005;99:S87-93.
- [Google Scholar]
- C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972-8.
- [Google Scholar]
- Angiotensin II and long-term arterial pressure regulation: The overriding dominance of the kidney. J Am Soc Nephrol. 1999;10(Suppl 12):S258-65.
- [Google Scholar]
- Regulation of glomerular filtration in essential hypertension: Role of abnormal Na transport and atrial natriuretic peptide. J Nephrol. 2002;15:489-96.
- [Google Scholar]
- The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167-74.
- [Google Scholar]
- Risk for chronic kidney disease increases with obesity: Health Survey for England 2010. Public Health Nutr. 2015;6:1-6.
- [Google Scholar]
- Fat mass gain predicts estimated GFR decline in a relatively healthy Korean population. Nephron Clin Pract. 2014;126:90-6.
- [Google Scholar]







