Translate this page into:
Baseline Anti-blood Group Antibody Titers and their Response to Desensitization and Kidney Transplantation
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In recent years, immunological barriers historically considered as absolute contraindications to transplantation are being reevaluated. One such barrier is the ABO blood group incompatibility. With better understanding of immunological mechanisms and effective various regimens for controlling it, ABO-incompatible (ABO-I) kidney transplantation is now being performed with increasing frequency. For good outcome, most important is to achieve and maintain low anti-blood group antibody titers (ABGATs). Twenty-two patients with ABO-I donors have been studied. The anti-A and anti-B antibody titers (IgG and IgM) were estimated by column agglutination technology using Automated Ortho BioVue System. For desensitization, pretransplant plasmapheresis and/or immunoadsorption and rituximab were used. ABGAT was determined before transplant and periodically after transplant. It was observed that one-third of the patients have low baseline ABGAT. In these cases with low ABGAT, transplant can be performed without any desensitization. In those with titers <1:256, rituximab (two doses of 200 mg weekly) and 3–6 sessions of plasmapheresis can bring down titers to <1:32. In those with titers >1:256, immunoadsorption may be used from the beginning to reduce ABGAT. After transplant, the titers drop to <1:8 in majority. Rise in titers to >1:64 require close observation and biopsy. If there is evidence of antibody-mediated rejection, treatment should be promptly started. Rise in titers 4–6 weeks after transplant is not associated with any graft dysfunction, and hence not of any clinical significance.
Keywords
Acute rejection
allograft biopsy
anti-blood group antibody titers
antibody-mediated rejection
desensitization
Introduction
The most effective treatment of end-stage renal disease is kidney transplantation, but a severe donor shortage has significantly limited this treatment. This problem is even more in countries with poor deceased donor transplant program and predominantly living related donor transplant program. To overcome this profound donor shortage, immunological barriers historically considered as absolute contraindications to transplantation are being reevaluated. One such barrier is the ABO blood group incompatibility.[1]
Paired exchange programs have assisted some of these recipients in undergoing transplantation. However, O recipients and AB donors remain at a disadvantage because O recipients can receive kidneys from group O donors only, whereas AB donors can donate to AB recipients only. In such cases, kidney transplantation across the ABO blood group barrier is the only option in a living donor transplant program. With better understanding of immunological mechanisms and various effective regimens for controlling it, ABO-incompatible kidney transplantation (ABO-I KT) is now being performed with increasing frequency.[23] Even in India, there are many centers now performing ABO-I KT.[456] However, the numbers are small, and there are very few published reports from India.
For good outcome, most important is to achieve and maintain low anti-blood group antibody titers (ABGAT).[7] We report our experience about ABGAT at baseline, after desensitization and after kidney transplant and the use of this knowledge in clinical practice.
Patients and Methods
Twenty-two patients with ABO-I donors have been studied. All combinations of ABO-incompatibilities were accepted including a two blood group antigen mismatch, that is, with donor AB and recipient O.
The IgG and IgM ABGAT were determined at baseline, during the desensitization process, and after kidney transplant. For decision-making, only IgG antibody titers were used. Plasmapheresis and/or immunoadsorption were attempted if baseline IgG ABGAT were >1:16 in the early period of our program and >1:32 in the later part of our program (after completing ten ABO-I transplants).
Detection of anti-A/B antibody titers
The anti-A and anti-B antibody titers (IgG and IgM) were estimated by column agglutination technology using Automated Ortho BioVue System.[8] This technique uses glass beads and reagent contained in a column of the cassette which, upon centrifugation, trap agglutinated red blood cells and allow nonagglutinated red blood cells to travel to the bottom of the column. The pooled “A” cell and “B” cell suspension (4%) were prepared fresh every day. The separated serum samples from patients were serially diluted for doubling titers with normal saline as 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, and 1:512,…etc. Reagent addition, sample addition, incubation (only for IgG), and centrifugation occur inside the automated instrument using software which also gives gradation readings of the agglutination reaction in the column well. The agglutination of RBCs was graded from 0 to 4+, and value of the highest serum dilution that gave a 1+ agglutination reaction was interpreted as the final titer.
Desensitization protocol
For desensitization [Figure 1], pretransplant, rituximab (200 mg) single dose was given on day-7. Tacrolimus (0.1 mg/kg), mycophenolate sodium salt (720 mg twice a day), and prednisolone 20 mg was started on the day rituximab was given. Plasmapheresis (alternate days) was also started a week before transplant. In those with high ABGAT (>1:256), immunoadsorption with Glycosorb column was tried in those who consented.
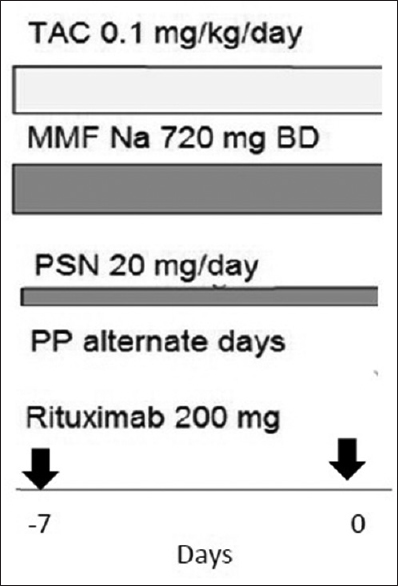
- Desensitization protocol - For patients with IgG anti-blood group antibody titers >1:256 at baseline immunoadsorption with Glycosorb column was tried
For plasmapheresis, one volume plasma exchange was done on alternate days starting 1 week before planned transplant. If the ABGAT did not drop to desired level, plasmapheresis was continued for additional 1 week. Replacement solution used was albumin.
Immunoadsorptions were carried out with the Glycosorb® ABO columns (Glycorex Transplantation AB, Lund, Sweden). These columns contain terminal trisaccharides with A or B Ag specificity, which are covalently bound to Sepharose™ particles. Standard procedure included 6 h session during which 18 L of plasma was treated. In one case, when titers did not drop to desired level, another session of immunoadsorption was carried out. When titers did not drop to desired level after 6 h, the process was continued for another 6 h.
Desensitization was considered successful if ABGAT dropped to <1:16 in the early period of our program and <1:32 in the later part of our program.
Induction and posttransplant immunosuppression
Patients who were successfully desensitized were taken up for transplant. Immunosuppression on the day of transplant-included rituximab 200 mg, thymoglobulin 1 mg/kg (single dose) or basiliximab 20 mg (repeated on day 4), and methyl prednisolone 500 mg.
Maintenance immunosuppression was tacrolimus 0.1 mg/kg in two divided doses (adjusted to maintain tacrolimus trough level 5–15 ng/ml), mycophenolate sodium 720 mg twice a day (adjusted as per white blood cell count), and tapering doses of prednisolone.
Monitoring of anti-blood group antibody titres
ABGAT was monitored on days after plasmapheresis (PP) or immunoadsorption during desensitization period. After transplant, it was monitored daily or on alternate days for 1 week, twice a week in the 2nd week, and weekly for next 4 weeks. Additional checking was done if clinically indicated. After 6 weeks, it was checked only if clinically indicated since accommodation occurs by 3–6 weeks.
Statistics
Data were analyzed using descriptive statistics. When the data were normally distributed, mean and standard deviation were calculated. When the data were not normally distributed, median and range were determined.
Results
Table 1 shows the characteristics of the patients. The mean age of recipients was 45 ± 11 years (age range, 10–58 years), 14 (63.6%) were men and 8 (35.4%) were women. Ten patients had O blood group, seven had A blood group, and five had B blood group.

The baseline IgG antibody titers ranged from 1:8 to >1:512 (median 1:64), whereas IgM antibody titers ranged from 1:2 to >1:512, (median 1:32). In almost all cases, IgM antibody titers were less than IgG antibody titers and as mentioned earlier, IgG antibody titers were considered for decision-making. Table 2 shows baseline ABGAT, preoperative ABGAT, and postoperative ABGAT of all patients.

The baseline ABGAT tended to be high in recipients with O blood group and lowest in those with B blood group. The baseline ABGAT in recipients with O blood group ranged from 1:64 to >1:512 (median 1:256). In recipients with A blood group, it ranged from 1:8 to >1:512 (median 1:64), and in recipients with B blood group, it ranged from 1:8 to 1:256 (median 1:32).
Response to desensitization
In 15 cases, plasmapheresis alone was attempted. In three of these cases (with baseline titer >1:512), the titers could not be reduced to <1:32. Based on this experience, we used Glycosorb with or without plasmapheresis in next three cases with high titers (one case with ABGAT 1:256 and 2 cases with titers >1:512). In one case with baseline ABGAT 1:256 and one with ABGAT >1:512, the titers dropped to 1:16 after single 6 h treatment. In one case with baseline ABGAT >1:512, the titers dropped to 1:64 with one session of 6 h. A second session of Glycosorb treatment was carried out after few days. When the titers did not drop below 1:64 after 6 h, the session was continued (total duration 12 h) until the titer dropped to 1:16. In one case with baseline ABGAT 1:8, no plasmapheresis or immunoadsorption was done. However, Rituximab was used.
Anti-blood group antibody titers after transplantation
Of the 19 patients who were transplanted, the titers remained <1: 64 (median 1:8) after transplant in 17. In two patients, IgG ABGAT rose to >1:64 in the 1st week after transplant. This was associated with 20% rise in creatinine above the lowest value achieved after transplant. Biopsy was done which was suggestive of acute antibody mediated rejection (ABMR). This was treated with PP, low dose IVIg (100 mg/kg after PP), rituximab (200 mg single dose), and bortezomib 2 mg on days 1, 4, 8, and 11). Both rapidly improved.
Discussion
Early attempts of kidney transplantation across the ABO blood group barrier led to a high rate of early graft loss because of humoral rejection.[9] The most important factor responsible for this was high ABGAT at the time of transplant and after transplant. Indeed, the results improved if transplants were performed after achieving low ABGAT and maintaining low levels after transplant. That is why we decided to analyze baseline ABGAT, the response to desensitization, and titers after transplantation.
We observed that the baseline ABGAT was low in many patients. The titers ranged from 1:8 to >1:512. In six patients, the titers at baseline itself were <1:32 [Table 1]. These patients could have been transplanted without any desensitization. Since it was the beginning of our ABO-I program, we aimed at titers <1:16 in the beginning and <1:32 in the later part to perform the transplant. Our observations suggest that patients should not be outright rejected for transplant just because the blood groups are incompatible. Transplant can be performed without any desensitization if baseline titers are <1:32. Indeed, in one patient with baseline titer 1:8, no desensitization was done before transplant.
The simplest and most common method to remove antibody from plasma is therapeutic plasma exchange, in which large amounts of plasma are withdrawn and replaced with colloid solutions.[10] This procedure eliminates approximately 20% of the anti-ABO antibodies with each session. However, this technique is not sufficiently selective to remove only blood group antibodies and also removes coagulation factors, hormones, and antiviral and antibacterial IgG and IgM. The removal of these factors increases the risk of bleeding and infection.[11]
In immunoadsorption, the plasma is processed through a Glycosorb ABO immunoadsorbent column and reinfused into the patient. There are no volume losses. It is very effective in reducing the antibody titers.[12] With about 18 L of plasma treated per 6 h session, antibody titers drop significantly. If the titers do not drop, the session can be continued longer as was done in one of our cases.
Most patients maintained titer below 1:32 during postoperative period as was observed by Shirakawa et al.[13] In two patients, the titer rose to 1:128. This was associated with clinical graft dysfunction and features of ABMR on biopsy. Both the patients were treated with plasmapheresis followed by IVIg, rituximab, and bortezomib and improved.
Some centers routinely perform immunoadsorption or PP after transplantation with a view to maintain ABGAT low after transplant. However, in our experience, majority of the patients maintain low ABGAT after transplantation and do not need plasmapheresis.
Conclusion
In summary, our study shows that almost one-third of the patients have low baseline ABGAT. In these cases with low ABGAT, transplant can be performed without any desensitization. In those with titers <1:256, rituximab (two doses of 200 mg weekly) and 3–6 sessions of plasmapheresis can bring down titers to <1:32. In those with titers >1:256, immunoadsorption may be used from the beginning to reduce ABGAT. After transplant, the titers drop to <1:8 in majority. Rise in titers to >1:64 requires close observation and biopsy. If there is evidence of ABMR, treatment should be promptly started. Rise in titers 4–6 weeks after transplant is not associated with any graft dysfunction and hence not of any clinical significance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Transplantation across previously incompatible immunological barriers. Transpl Int. 2006;19:87-97.
- [Google Scholar]
- The critical role of plasmapheresis in ABO-incompatible renal transplantation. Transfusion. 2008;48:2453-60.
- [Google Scholar]
- Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4:1315-22.
- [Google Scholar]
- ABO incompatible kidney transplantation e a single center experience. Indian J Transplant. 2012;6:103-6.
- [Google Scholar]
- 2014. 9-Year-Old Gets Mum's Kidney. Available from: http://www.dnaindia.com/mumbai/report-9-year-old-gets-mom-s-kidney-2009260
- Overcoming the ABO incompatibility barrier in pediatric renal transplantation. Indian Pediatr. 2015;52:704-6.
- [Google Scholar]
- ABO-incompatible solid-organ transplantation. Am J Clin Pathol. 2006;125(Suppl):S87-94.
- [Google Scholar]
- The BioVue column agglutination technology test: A new way to detect red cell antigen-antibody reactions. Transfusion. 1990;30:109-13.
- [Google Scholar]
- Renal homografts in patients with major donor-recipient blood group incompatibilities. Surgery. 1964;55:195-200.
- [Google Scholar]
- ABO-incompatible kidney transplantation: Current practice and the decade ahead. Curr Opin Organ Transplant. 2010;15:526-30.
- [Google Scholar]
- ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145-8.
- [Google Scholar]
- The low dose of rituximab in ABO-incompatible kidney transplantation without a splenectomy: A single-center experience. Clin Transplant. 2011;25:878-84.
- [Google Scholar]







