Translate this page into:
De Novo Collapsing Glomerulopathy in Renal Allograft in Association with BK Virus Nephropathy in a Child and Stabilization of Renal Function by Elimination of Viremia
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Well-recognized association between HIV 1 infection and collapsing glomerulopathy (CG) raises the possibility that intrarenal infection by other viruses may also contribute to the development of this lesion in native or post-transplant kidneys. There is evidence in literature about association of these lesions with cytomegalovirus, Epstein–Barr virus, hepatitis C virus, and parvovirus B19 infections. Here, we present a case report of post-transplant BK virus nephropathy in a male child who was found to have CG in subsequent biopsy 2 months later. His renal function and proteinuria were stabilized on elimination of viremia.
Keywords
BK viremia
collapsing glomerulopathy
renal allograft
sv40
Introducion
Weather collapsing glomerulopathy (CG) is a variant of focal segmental glomerulosclerosis (FSGS) or distinct pathological entity remains to be understood. HIV associated nephropathy with morphology of CG is well described in native kidney. It raises the possibility than other viral infections and viral gene products may contribute to this pathological development in native or post-transplant kidneys. Here we report a first post-transplant case of BKV nephropathy who on sequent biopsy showed CG. His renal function and proteinuria did not deteriorated further once viremia was eliminated by reducing immunosuppression.
A 13-year-old male child weighing 30 kg (body mass index 19.2 kg/m2) presented to us with complaints of easy fatigability, anorexia, and weight loss of 3–4 kg during the last 2 months. There was no history of hematuria or edema on the face or feet. There was no history of hospitalization in the past. His motor and psychosocial milestones were normal in early childhood, and he had satisfactory scholastic performance. There was no history of renal disease in family members. His urine output was 800–1000 ml/day. There was no apparent bony deformity. His blood pressure was 130/84 mm Hg. On investigation, hemoglobin was 9.0 g/dl, blood urea 120 mg/dl, serum creatinine 7.0 mg/dl, sodium 138 mEq/L, potassium 5 mEq/L, calcium 9.8 mg/dl, phosphate 5.4 mg/dl, serum albumin 2.8 g/dl, serum cholesterol 180 mg/dl, alkaline phosphatase 410 U/L, intact parathyroid hormone 530 ng/L, and venous bicarbonate 14 mEq/L. He was negative for hepatitis B surface antigen (HBsAg), HIV, and hepatitis C virus (HCV) by ELISA. Cytomegalovirus (CMV) IgG was positive. Urine examination showed trace urine albumin and normal sediments. Twenty-four-hour urine protein was 250 mg. Urine culture was negative. His sonography of the abdomen showed only one kidney in the right renal fossa measuring 4.5 cm × 2 cm with loss of corticomedullary differentiation. His voiding cystourethrogram was normal. Provisional diagnosis of tubulointerstitial disease was made.
He underwent living-related renal transplant after being on maintenance hemodialysis for 2 months at our institute. His 55-year-old grandmother was a donor (5/6 A, B, DR mismatch). CMV status was D+/R+. The child received pulse methylprednisolone of 250 mg on day 0, 1, and 2 along with rabbit antithymocyte globulin 50 mg single dose as an induction agent. Subsequently, he was put on 20 mg prednisolone, tacrolimus 1 mg twice a day (0.07 mg/kg), and mycophenolate sodium 360 mg twice a day along with cotrimoxazole DS once a day and valganciclovir 450 mg once a day. He had uneventful immediate postoperative course. He was discharged on the 8th post-transplant day with serum creatinine 0.8 mg/dl (tacrolimus trough level 9.5 ng/ml). His steroid dose was tapered gradually, and at the end of 3 months, he was on 5 mg prednisolone, tacrolimus 1 mg twice a day, and mycophenolate sodium 360 mg twice a day. Subsequently, his graft function remained stable for 1 year. His BK viremia (BKV) polymerase chain reaction (PCR) in blood was performed at 1, 3, 6, and 9 months and it was undetectable. After 1st year of transplant, he had asymptomatic rise in serum creatinine from 1.0 mg/dl to 1.6 mg/dl. His serum albumin was 2.9 g/dl and serum cholesterol was 290 mg/dl. His urine examination showed ++ proteinuria along with plenty of decoy cells. His 24 h urinary protein was 1.2 g. Graft Doppler revealed normal color flow and normal resistive index (<0.8). His blood BKV PCR was positive with 15,800 copies/ml. His donor-specific antibody test (DSA) was negative. Percutaneous graft biopsy was performed under local anesthesia. It revealed normal glomeruli with mild mesangial prominence. Some of tubular cells had enlarged nuclei with intranuclear inclusions having ground glass appearance and interstitium filled with diffuse mononuclear cell infiltration with admixed polymorphonuclear cells. Blood vessels and peritubular capillaries (PTCs) were unremarkable for tubular injury. C4d staining was negative and sv40 staining was positive by immunohistochemistry [Figure 1]. Hence, considering BKV nephropathy, immunosuppressant drug regimen was modified. Mycophenolate was discontinued and switched to leflunomide (10 mg twice a day). Tacrolimus was switched to cyclosporine 50 mg twice a day and prednisolone was continued in low dose (5 mg). BKV PCR in blood was followed every month. Over 2 months, serum creatinine crept from 1.6 to 2.3 mg/dl and BKV PCR blood was 6000 copies/ml. Hence, the second biopsy [Figure 2a and b] was performed. It consisted of ten glomeruli with collapse of capillary tuft in two glomeruli. One glomerulus had segmental collapse while the other had global collapse of the tuft with surrounding swollen visceral epithelial cells, leading to pseudocrescent formation. Some of tubular cells had enlarged nuclei with intranuclear inclusions having ground glass appearance, and interstitium showed moderate fibrosis with diffuse mononuclear and polymorphonuclear cell infiltration. Blood vessels and PTCs were unremarkable, C4d staining was negative, and sv40 was still positive by IHC. DSA was also negative. We investigated to find out other known causes for collapsing glomerulopathy (CG). Parvovirus B19 DNA, Epstein–Barr virus (EBV), and CMV PCR and ELISA HIV, HBsAg, HCV were negative. He had no history of interferons, bisphosphonates, or valproic acid intake. His BKV PCR in the blood was followed monthly, and it became undetectable after 4 months. After 6 months of the first biopsy, his serum creatinine stabilized at 2.4 mg/dl (that is, it did not deteriorated after the second biopsy when BKV load was decreasing without requiring further reduction of immunosuppression) and his 24 h urine protein was 600 mg on immunosuppressive regimen of cyclosporine and prednisolone.
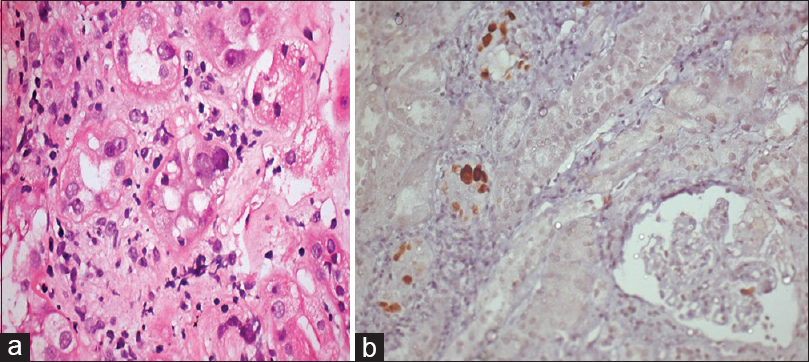
- (a) Tubular cells having enlarged, basophilic, ground glass nuclear inclusions with mixed inflammatory cells in the interstitium (H and E, ×200). (b) Tubular cells with sv40 positive viral inclusions (sv40 IHC, ×200)
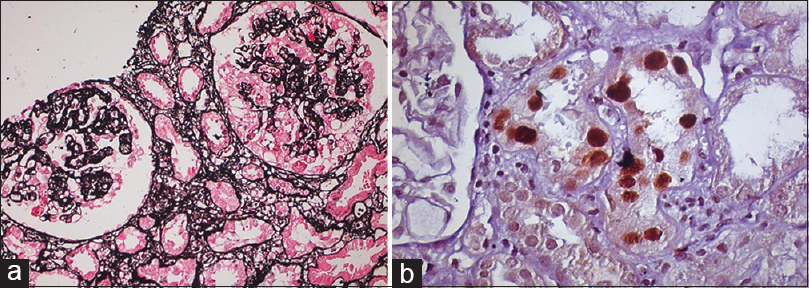
- (a) Two glomeruli, one of which shows collapse of the capillary tuft with proliferation of visceral epithelial cells (JSM, ×200). (b) Tubular cells having enlarged, basophilic, ground glass nuclear inclusions showing sv40 positively (sv40, ×400)
Discussion
The term “CG” was first described by Weiss et al. to describe a distinct entity with progressive renal failure and renal pathological features characterized by segmental or global capillary collapse and visceral epithelial cell swelling and hyperplasia producing pseudocrescent and extensive tubulointerstitial inflammation.[1] Whether CG is a variant of focal segmental glomerulosclerosis (FSGS) or a distinct pathological entity remains to be understood. However, it is believed to be more aggressive form of disease as compared to classical form of FSGS.[23] Human immunodeficiency virus-associated nephropathy with morphology of CG is well described in native kidneys.[4] It raises the possibility that other viral infections and viral gene products may contribute to this pathological development and manifestations.
There are case reports of CG in association with acute CMV infection with viremia and IgM antibodies or CMV DNA in renal biopsy by PCR, either in native or transplanted kidney.[567] Presne et al. showed improvement in association with ganciclovir therapy along with steroid.[5] Grover et al. reported CMV-induced CG in native kidney and showed patient becoming dialysis independent after ganciclovir treatment.[7] Laurinavicius et al. in clinicopathological study of CG in HIV and non-HIV patients found CG in three HCV-positive patients out of 42 HIV-negative patients.[4]
Recently, Joshi et al. reported a case of young woman having acute EBV infection who developed collapsing FSGS.[8] Moudgil et al. reported parvovirus B19 infection with collapsing GN.[9]
Here, we report a first case of proved post-transplant BKV nephropathy in a 13-year-old child who was subjected to repeat renal biopsy to rule out associated rejection after 2 months of first biopsy for unsatisfactory improvement despite reduction of immunosuppression. To our surprise, biopsy showed glomerular tuft collapse in 2/10 glomeruli with pseudocrescent. On IHC, sv40 was positive in both biopsies. At the time of repeat biopsy, viremia was still present although copies were reduced. Viral copies were undetectable in the blood after 4 months. Follow-up showed stabilization of renal function and reduced proteinuria after 6 months of diagnosis.
Since it is a first case report showing CG on subsequent biopsy of BKV nephropathy, it remains to be clarified whether it is nonspecific morphological feature of progressive kidney damage or it is because of altered glomerular cell biology, by particular viral infection or viral gene product.
Conclusion
To best of our knowledge, this is the first case report showing association of BKV infection with CG and also showing stabilization of function after elimination of viremia. As our understanding of other genetic and reactive reasons causing CG increases, it may suggest possible therapeutic targets to control the progression of CG by therapeutic intervention.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nephrotic syndrome, progressive irreversible renal failure and glomerular ‘collapse’: A new clinico-pathologic entity. Am J Kidney Dis. 1986;7:20-8.
- [Google Scholar]
- Collapsing glomerulopathy: A clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int. 1994;45:1416-24.
- [Google Scholar]
- Collapsing glomerulopathy in renal allografts: A morphological pattern with diverse clinicopathologic associations. Am J Kidney Dis. 1999;33:658-66.
- [Google Scholar]
- Collapsing glomerulopathy in HIV and non-HIV patients: A clinicopathological and follow-up study. Kidney Int. 1999;56:2203-13.
- [Google Scholar]
- Collapsing glomerulopathy and cytomegalovirus, what are the links? Presse Med. 2000;29:1815-7.
- [Google Scholar]
- Acute cytomegalovirus infection complicated by collapsing glomerulopathy. Nephrol Dial Transplant. 2003;18:187-9.
- [Google Scholar]
- Cytomegalovirus-induced collapsing focal segmental glomerulosclerosis. Clin Kidney J. 2013;6:71-3.
- [Google Scholar]
- Acute Epstein-Barr virus infection-associated collapsing glomerulopathy. Clin Kidney J. 2012;5:320-2.
- [Google Scholar]
- Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int. 2001;59:2126-33.
- [Google Scholar]







