Translate this page into:
Prevalence and Clinical Correlates of White Coat Effect in Patients with Chronic Kidney Disease and the Role of Automated Blood Pressure Device in its Assessment
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Context:
Hypertension in chronic kidney disease (CKD) is an important modifiable cardiovascular risk factor. Patients with CKD can have clinically significant white coat effect (WCE), making routine clinic blood pressure (BP) measurements an unreliable indicator of actual BP control. Automated BP monitoring is useful in identifying WCE. The utility of automated BP monitoring has seldom been part of clinical practice in developing countries.
Aim:
The goal of this study was to estimate the prevalence and determinants of WCE in adult patients with CKD in an outpatient setting using an automated BP device.
Materials and Method:
In this prospective observational study, patients with CKD attending the nephrology clinic over a period of 6 months (January 2016 to July 2016), who were suspected to have WCE by the treating physician, were assigned to measurement of BP by both the standardized manual BP recording by a single nephrologist and with automated machine as per a defined protocol. Clinical, demographic characters that would influence outcomes were also studied.
Results:
Among 118 patients with CKD with suspected WCE, 57.6% showed WCE. The mean systolic and diastolic BPs were significantly lower with automated machine when compared with manual BP recordings in patients with WCE (p = 0.04). WCE was seen in all stages of CKD. Occurrence of WCE in CKD was not dependent on factors such as old age, sex, diabetes mellitus, or smoking status in our study.
Conclusion:
WCE is a highly prevalent and underdiagnosed entity in patients with CKD. Automated machine is a useful and time-saving tool in detection of WCE in patients with CKD attending the outpatient clinic and guide management.
Keywords
Automated machine device
chronic kidney disease
hypertension
white coat effect
Introduction
The term white-coat hypertension (WCH) was coined by Thomas Pickering. It is also referred to as “office hypertension” or “isolated clinical hypertension.”[1] It is a term classically used to denote individuals who were not on treatment for hypertension (HTN), with elevated office blood pressure (BP), and normal BP measured outside the medical setting. White coat effect (WCE), on the other hand, is high BP associated with clinic visit compared with ambulatory BP monitoring (ABPM) or home readings in patients who are on treatment for HTN.[2] Its clinical importance is related to the possibility that it might lead to overestimation of the initial BP levels and/or to underestimation of the effect of antihypertensive treatment. Although definition wise they are different, both WCH and WCE are like two faces of the same coin. The overall incidence of WCE in the general population has been varied in epidemiological studies with incidence ranging from 10.4% to 52.9% in population-based surveys.[3]
Chronic kidney disease (CKD) and HTN are conditions which often coexist. Incidence of HTN has been found to be nearly 80%–90% in patients with CKD.[4] Uncontrolled BP has been time and again proven to be associated with progression of CKD and is also a major risk factor for cardiovascular related death, which is the most common cause for mortality in patients with CKD.[567] The prevalence of WCE is high in patients with CKD accounting up to 30%.[8] It is a significant confounder in evaluation and management of patients with HTN in patients with CKD considering the large percentage of patients with HTN.[9] Overestimating BP based on manual office recordings as a consequence of WCE has medical, economical, and medico legal consequences. Accurate estimation of BP is therefore paramount in the management of patients with CKD.
The 24-h ABPM remains the “gold standard” for diagnosis of HTN.[10] However, using 24-h ABPM for every visit or follow-up maybe cumbersome and not feasible considering time and cost constraints. A number of office-based automated BP monitoring systems have been developed in the world, one among which is BpTRU. It has been developed by VSM MedTech Ltd. (Coquitlam, BC, Canada) specifically designed for clinician's office. The correlation between BpTRU reading and 24-h ABPM has already been established in both normal and CKD population.[1112] However, despite the correlation shown in randomized control trials, the utility of automatic BP monitoring has seldom been part of clinical practice in developing countries. There are no studies in India/Asia which have highlighted the utility of this office-based method in diagnosis and management of WCE especially in population of patients with CKD where optimal BP management is often the game changer for the patients. The purpose of this study is to determine the prevalence of WCE in patients with CKD and possible clinical utility of automated BP monitor in diagnosis and monitoring of HTN in patients with CKD in an outpatient nephrology clinic at a tertiary care center.
Materials and Methods
This is a single-center prospective observational study done in a nephrology outpatient department (OPD) over 6 months duration from January 2016 to July 2016. Ethical committee clearance was taken. All patients were informed about the study protocol and gave their written informed consent before study enrolment.
In all, 118 consecutive patients with CKD attending the nephrology OPD who had uncontrolled BP as per clinic BP readings and suspected to have WCE (as defined in inclusion criteria) were studied. BpTRU machine (model BPM-200) [Figure 1] was used as a tool for identification of WCE.

- BpTRU machine. Courtesy – (BpTRU medical devices) (www.bptru.com/products)
CKD was defined and staged as per National Kidney Foundation, Kidney Disease Outcomes Quality Initiative guidelines (NKF KDOQI).[13] eGFR was calculated as per the Modification of Diet in Renal Disease formula.[14]
Inclusion criteria: Adult (>18 years of age) patient with CKD and HTN who has any one of the following during his routine visit to nephrology clinic:
-
Office/clinic BP >140/90 mmHg on at least three separate clinic/office visits with two separate measurements made at each visit in spite of antihypertensive treatment
-
Clinic BP >140/90 mmHg, and at least two home BP measurements taken outside the office which are <140/90 mmHg suggestive of having WCE were included for the study.
Exclusion criteria: Patients with sepsis, fever, and hypertensive emergency and pregnant patients.
An initial reading was taken by a single nephrologist with a standard manual mercury sphygmomanometer, after which the patient's BP was measured with BpTRU device as per protocol.
BpTRU device
BpTRU is an automated device manufactured by VSM MedTech (Coquitlam, BC, Canada) that takes serial BP measurements for use in a physician's office. It works on the principle of oscillometry. The device uses a BP cuff with an automated inflation and deflation mechanism. The cuff measures oscillations in the pulses in the upper arm and uses a computer algorithm to calculate the systolic and diastolic BP. Usually, the initial reading is taken while a physician or nurse is present and then discarded. Five additional measurements are subsequently taken at intervals of 1–5 min while the patient is alone in a room. These measurements and the average of the last five readings are displayed to the nearest 1 mmHg. BpTRU device has been validated for accuracy by the British Hypertension Society and the Association for the Advancement of Medical Instrumentation.
Measuring BP with BpTRU
The following protocol was adopted in our study:[11]
-
The subjects remained seated for at least 5 min
-
The non-dominant hand was used to tie BpTRU cuff by a trained nurse
-
The initial reading was taken by the nurse and discarded
-
The patient was then left alone in the room and BpTRU device took a minimum of five readings at intervals of 2 min.
These five readings were averaged by the device and this average was recorded.
Definition of WCE
Based on comparison between automatic BP reading and clinic BP, WCE was defined as having an office BP of at least 20 mmHg systolic and/or 10 mmHg diastolic higher than automatic BP readingt.[15]
Clinical characteristics of the patients including age, gender, etiology, stage of CKD and comorbidities such as presence of diabetes mellitus (DM) and smoking status were recorded. The patients were also grouped according to different stages of CKD as per KDOQI guidelines of 2003.[13] The prevalence of WCE and its determinants and clinical utility of automated BP monitor in the diagnosis in patients with CKD were studied.
Statistical analysis
The data were analyzed using SPSS version 15.0. Descriptive statistics were calculated for all patients involved in the study. Continuous variables are reported as mean ± standard deviation throughout where normally distributed. Chi-square test was applied for analysis of results between stages of CKD vs WCE, smoking vs WCE, DM vs WCE, with p value less than 0.05 considered as statistically significant. The paired Student's t-test was used for analysis of significant differences between routine clinic and automatic BP monitoring with p value less than 0.05 considered as statistically significant. The change in automatic BP readings from first reading to fifth reading was analyzed using repeated measures of ANOVA with p value less than 0.05 considered as statistically significant.
Results
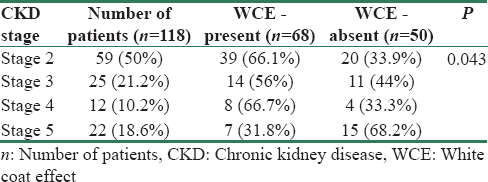
A total of 118 patients were included in the study. Of the 118 patients, 69 were males (58.5%) with mean age of 43.94 ± 14.93 years. WCE was showed by 57.6% (68 of 118) patients. The most common cause for CKD in our study population was DM (45%). About 26% of patients were smokers in the study. Patients with CKD stage 2 comprised nearly 50% of the study population. The distribution of patients as per CKD stage is as shown in Table 1.
In our study, the measurements were taken with manual readings using mercury manometer (156.32 ± 15.6/95.26 ± 9.4 mmHg) were significantly higher compared with average automatic BP reading (132.35 ± 13.2/84.47 ± 8.6 mmHg) (p < 0.001).
In patients with WCE, the average automatic BP readings (123.53 ± 13.8/78.51 ± 7.9 mmHg) compared with clinic BP (160.38 ± 14.7/95.59 ± 8.3 mmHg) showed a markedly higher systolic BP by 33 mm Hg and diastolic BP by 17 mmHg (p < 0.001), whereas in patients without WCE it was higher by 9 mmHg systolic and 2 mmHg diastolic (p = 0.34), respectively [Figures 2 and 3]. Also in patients with WCE, the automatic BP readings showed a significant downward trend from first automatic BP reading to fifth reading [Figure 4] compared with patients with no WCE.

- Mean systolic blood pressure (mmHg) (with standard deviations) in patients with and without white coat effect

- Mean diastolic blood pressure (mm of Hg) (with standard deviations) in patients with or without white coat effect

- Serial automated recordings in patients with and without WCE with average automated readings
When we looked into the presence of WCE, 68 patients showed WCE (57.6%) in our study accounting for higher prevalence in our CKD population. Another important finding was 53 of 68 patients (77.9%) who showed WCE had BP <140/90 mm Hg in and thus did not require any dose adjustment for antihypertensives unnecessarily based on manual BP. This emphasizes the role of using automated BP reading in clinical setting to eliminate WCE. In our study, WCE was seen in all stages of CKD. In patients with CKD stage 2, 66.1% of patients had WCE, stage 3 had 56%, stage 4 had 66.7%, and stage 5 had 31.8% as shown in Table 1. Compared to other stages in CKD, stage 5 had less prevalence of WCE in our study (p = 0.043).
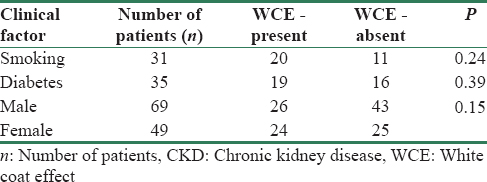
The distribution of WCE was similar in both males and females (p = 0.15). Among patients with DM, 18 (52.9%) of them showed WCE. When we compared the diabetics with non-diabetics, there was no difference in incidence of WCE (p = 0.39). There was also no increased occurrence of WCE in smokers (p = 0.24) [Table 2].
Discussion
HTN is present in 90% of patients with CKD and is the most important modifiable risk factor.[6161718] Optimal BP control is a main treatment goal to improve renal and cardiovascular prognosis in patients with CKD. WCE may, in part, account for the strikingly high prevalence of uncontrolled HTN in patients with CKD due to the suboptimal assessment of BP with clinic BP measurements alone despite regular follow-up in tertiary care and multidrug antihypertensive therapy. The majority of cases management is based on a single clinic BP reading which may not reflect the true BP.[6] Automated BP monitoring has been used to detect WCE in general population and also in patients with CKD and is better correlated to day-time average ABPM as proved beyond doubt in previous studies.[1112] In study by Brothwell et al., there was no significant detectable difference between automated BP readings and the day-time mean of 24-h ABPM in patients with CKD.[11]
In our study, we used automated BP recording to see the prevalence of WCE in patients with CKD attending nephrology clinic at a tertiary care center. The prevalence was high with 56.7%. Our study highlights the existence of high prevalence of WCE in our patients with CKD. In previous studies, the prevalence has ranged from 10.5% to 31.7%. Minutolo et al. from Italy showed a prevalence of 30% of WCE in CKD.[8] Similarly, the meta-analysis by Bangash et al. showed a prevalence of 30% (95% CI 26.5 to 33.5%) in patients who had HTN in the clinic.[19] In our study, there is a significant prevalence of WCE; almost one patient out of two had WCE. There is substantial heterogeneity between all these studies. The prevalence of WCE in CKD varies partly due to different cut-offs being used for diagnosis, different techniques, and due to a plethora of factors that may influence its presence. The high occurrence in our study may be due to the measurement done by nephrologist and due to demographic heterogeneity.
In a study by Myers et al. in hypertensive patients, the mean of five automated measurements was significantly lower (142 ± 21/80 ± 12 mmHg) than readings taken with a standard mercury manometer (163 ± 23/86 ± 12 mmHg) (p < 0.001). In their study, the mean BP in the automated group was reduced by 13.9 (systolic)/3.7 (diastolic) mmHg, compared to the manual office BP which is similar to our findings.[20] Similarly, Brothwell et al. in patients with CKD also showed that the average automated systolic and diastolic readings (117.3 ± 14.1/78.4 ± 10.0 mmHg) were significantly lower than the routine clinic readings (143.8 ± 15.5/82.4 ± 11.2 mmHg) (p < 0.001) emphasizing the fact of using automated readings in clinical practice for better monitoring of HTN.[11]
Previous studies have highlighted the prevalence of WCE in general population and in patients with CKD and modification in management by introduction of automated office-based sphygmomanometers.[1220]
The distribution of WCE is prevalent across all the stages of CKD highlighting the importance in using automated BP instrument in our clinical practice for detection of WCE in CKD. However, in our study, the prevalence of WCE in stage 5 CKD was significantly less than other stages. The probable reason could be due to the fact that a majority of our stage 5 CKD population had been on follow-up for a longer period of time, thus eliminating any significant WCE. However, a larger study population may be required to support this finding. Since we identified the presence of WCE, it helped in not uptitrating antihypertensive medication in 44.9% of patients (53 of 118) unnecessarily.
Lindbaek et al. in their study regarding the predictors of systolic WCE in patients with suspected and treated HTN analysis revealed a higher mean BP, age, smoking, and family history of cardiovascular disease as predictors of WCE.[21] Mansoor et al. reported that increasing age was associated with an increase in the level of WCE.[22] In a study by Huang et al. in non-diabetic hypertensive patients, only female gender predicted WCE occurrence.[23] In a systematic review by Sheppard et al., female sex was the only significant predictor of WCH (odds ratio 3.38, 95% confidence interval 1.64–6.96).[24] Our study compared gender, age, smoking. and presence of DM as predictive factors for WCE. However, no association was found with gender, age, smoking, or presence of DM. Since most studies mentioned above were done in a population with hypertensives with predominantly normal renal functions, the role of these factors in patients with CKD is a matter which requires further research.
Automated device is routinely used in clinical practice in Canada, Europe, and the United States. The recently concluded CAMBO trial (The Conventional versus Automated Measurement of Blood pressure in the Office) in hypertensive patients has demonstrated clearly that use of automated device virtually eliminates white coat response experienced by many patients when manual BP readings are recorded in routine clinical practice.[25] It is being validated in all ethnics population in previous studies.[2627] The use of automated device is not much in developing countries like us. Ours is the first study in South East Asia and it shows significant prevalence of WCE in patients with CKD attending nephrology outpatient clinic in our region and utility and importance of using simple automated device in identifying it and thus preventing unnecessary uptitration of medications in our routine clinic practice. It is evident that the average of five automated BP measurements, taken when the patient is alone, more reliably reflects “resting” BP compared with manual BP taken with a stethoscope and sphygmomanometer. Automated device can improve HTN management by replacing conventional manual BP measurements, which are often poorly performed and inaccurate.[272829]
However, there are some limitations in our study. It is a single-center study with small study group. In our study, we did not use 24-h ABPM which is considered the gold standard to confirm WCE. However, the widespread use of ABPM in routine clinical practice is impractical, because it is expensive, time-consuming, and not always available.
Conclusion
The prevalence of WCE is very high in patients with CKD. Smoking, diabetes, and gender does not have any significant impact on WCE in patients with CKD. The use of automatic clinic BP device in CKD clinics helps in effectively diagnosing WCE and monitoring HTN. It avoids unnecessary up titration of antihypertensive therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank their nephrology clinic staff and patients.
References
- Prevalence of white-coat and masked hypertension in national and international registries. Hypertens Res. 2014;38:1-7.
- [Google Scholar]
- Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1-12.
- [Google Scholar]
- Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-305.
- [Google Scholar]
- Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:406-11.
- [Google Scholar]
- Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: Prospective population based cohort study. BMJ. 2010;341:c4986.
- [Google Scholar]
- Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171:1090-8.
- [Google Scholar]
- European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821-48.
- [Google Scholar]
- Optimising the accuracy of blood pressure monitoring in chronic kidney disease: The utility of BpTRU. BMC Nephrol. 2013;14:218.
- [Google Scholar]
- The BpTRU automatic blood pressure monitor compared to 24-h ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5:18.
- [Google Scholar]
- National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med. 2003;139:137-47.
- [Google Scholar]
- A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-70.
- [Google Scholar]
- Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med. 2008;121:332-40.
- [Google Scholar]
- A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305:1553-9.
- [Google Scholar]
- Importance of blood pressure control in chronic kidney disease. J Am Soc Nephrol. 2006;17:S98-103.
- [Google Scholar]
- Masked hypertension and white-coat hypertension in chronic kidney disease: A meta-analysis. Clin J Am Soc Nephrol. 2009;4:656-64.
- [Google Scholar]
- Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280-6.
- [Google Scholar]
- Predictors for the white coat effect in general practice patients with suspected and treated hypertension. Br J Gen Pract. 2003;53:790-3.
- [Google Scholar]
- Determinants of the white-coat effect in hypertensive subjects. J Hum Hypertens. 1996;10:87-92.
- [Google Scholar]
- Clinical predictors of significant white-coat effect in non-diabetic hypertensive patients. Acta Cardiol Sin. 2010;26:151-6.
- [Google Scholar]
- Predictors of the home-clinic blood pressure difference: A systematic review and meta-analysis. Am J Hypertens. 2016;29:614-25.
- [Google Scholar]
- Conventional versus automated measurement of blood pressure in the office (CAMBO) trial. Fam Pract. 2012;29:376-82.
- [Google Scholar]
- Ethnicity and differences between clinic and ambulatory blood pressure measurements. Am J Hypertens. 2015;28:729-38.
- [Google Scholar]
- BPTRU – An automated blood pressure monitoring device in the physician's office – A useful alternative to 24 h ambulatory blood pressure monitoring in the evaluation and management of hypertension in India. Int J Cardiol. 2009;137:S17-8.
- [Google Scholar]
- In-office assessment of blood pressure in chronic kidney disease: Usual measurement versus automated BpTRU measurement. Blood Press Monit. 2011;16:124-8.
- [Google Scholar]
- Evaluation of the overall efficacy of the Omron office digital blood pressure HEM-907 monitor in adults. Blood Press Monit. 2001;6:107-10.
- [Google Scholar]







