Translate this page into:
Post-Renal Transplant Metabolic Acidosis: A Neglected Entity
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Metabolic acidosis is a prevalent yet overlooked entity among renal transplant recipients (RTRs) and incurs adverse effects on graft function. Although graft dysfunction and calcineurin inhibitor usage have been linked with renal tubular acidosis (RTA), there is no Indian data on prevalence or risk factors of post-transplant acidosis. A cross-sectional study was conducted on 106 adult RTRs, with a transplant duration of >6 months and an estimated glomerular filtration rate (GFR) >40 ml/min/1.73 m2. Acidosis was diagnosed on basis of plasma bicarbonate and arterial pH. Serum and urine electrolytes with anion gap were determined to diagnose and type RTA. Acidosis was diagnosed in 44 of 106 patients (41.5%) with 23 (52.27%) having severe acidosis. Type I RTA was the most common subtype (52.5%) followed by type IV (30.9%) and type II RTA (7.5%). The correlation between estimated glomerular filtration rate and acidosis was minimally linear (r = 0.1088), with multivariate analysis revealing previous acute rejection episodes, current serum tacrolimus levels, cotrimoxazole usage and intake of animal proteins to be independent risk factors. The serum albumin levels were low in the acidosis group and showed linear correlation with bicarbonate levels (r = 0.298). There is a high prevalence of metabolic acidosis in RTRs with type I RTA being most common subtype. Screening of RTRs on a regular basis is a feasible approach for early diagnosis and intervention. However, prospective studies are needed to demonstrate the effect of acidosis on graft survival and benefit of bicarbonate therapy in RTRs.
Keywords
Drugs
metabolic acidosis
renal transplant
renal tubular acidosis
Introduction
Metabolic acidosis post-renal transplant, an entity reported as early as 1967, is a common and significant clinical problem yet under looked.[1] Routine assessment of this basic metabolic disorder is not widely practised and the treatment of metabolic acidosis is not established in renal transplant recipients (RTRs) as well. This can be inferred indirectly by the absence of reference to this issue in 2009 – Kidney Disease: Improving Global Outcomes guidelines for the care of KTx recipients.[2] The real prevalence of metabolic acidosis is a difficult figure to establish as very few papers have assessed the issue in quite small population; ranging from 13% to over 50%.[345] However, considering that most of the studies included KTx patients with glomerular filtration rate (GFR) values over 40 ml/min, this figure is consistently higher than that reported for other non-KTx CKD cohorts with comparable degrees of renal function.[6]
Till date, there is no Indian data available addressing the above issue in kidney transplant recipients. Identification and correction of metabolic acidosis might contribute to graft survival.
In this background we aimed
-
To assess the prevalence of metabolic acidosis in RTRs
-
To assess the type, severity and risk factors of metabolic acidosis in RTRs
-
To determine the cut-off of serum tacrolimus trough (C0) levels associated with acidosis in RTRs.
Materials and Methods
This is a cross-sectional study of adult renal allograft transplant recipients (RTRs) under outpatient follow-up in our centre who underwent kidney transplantations between January 2013 and December 2016. We screened 170 patients in total, including living related and deceased donor RTRs with a minimum post-transplant duration of 6 months and estimated GFR (eGFR) >40 ml/min/1.73 m2. We excluded patients with eGFR <40 ml/min/1.73 m2, unstable graft function, recent acute rejection (<6 months), any active infection and diarrhoea. Our final study population was 106 RTRs with stable graft function on conventional maintenance immunosuppression with steroids, tacrolimus and mycophenolate mofetil. All deceased donor RTRs had received rabbit ATG-based induction, spousal donor transplant recipients received basiliximab as induction agent while recipients of living-related donor kidneys had not received any induction therapy. As a part of unit's transplant protocol, all patients were given valganciclovir prophylaxis for 3 months and cotrimoxazole prophylaxis for life. All patients gave written informed consent and no expenditure was incurred by the patient for this study. The study was approved by the institutional ethics committee.
The basic demographic profile, medical and drug history were obtained from medical records. Estimated GFR was calculated using the Modification of Diet in Renal Disease (MDRD-4) equation. All patients underwent analysis of serum electrolytes including serum bicarbonate (by ISE) with serum albumin and arterial blood gas analysis (by 121 COBAS analyser). The patients with metabolic acidosis were further analysed for urine pH, urine anion gap (UAG) and serum anion gap (SAG), which were formulated as follows: UAG = [Na+] + [K+] – [Cl−] and SAG = [Na+] – [Cl− + HCO3−]. SAG value between 7 and 14 was accepted to be normal. Serum tacrolimus trough levels (C0) were measured by chemiluminescence immunoassay (CLIA). Urine culture and sensitivity was done for all patients to remove the confounding effect of asymptomatic urinary tract infection.
Metabolic acidosis was defined as serum bicarbonate <22 mEq/L with pH <7.35 and severe acidosis as serum bicarbonate <20 mEq/L. Renal tubular acidosis (RTA) was defined to be metabolic acidosis with normal SAG and positive UAG. The patients with RTA were further differentiated into type I distal RTA if urine pH was >5.5 and serum potassium was low or normal, type IV if urine pH was >5.5 and serum potassium was high or type II RTA if urine pH was <5.5 and serum potassium was low or normal.
Statistical analysis
Data were analysed using the rv.2.4.2 software. All categorical data were summarised using frequency and percentages, all continuous data were described using mean and standard deviation or median and interquartile range based on the distribution. To study the risk factors of acidosis, univariate logistic regression was applied and factors which were significant >10% level were included for multivariate logistic regression. Independent sample T-test was applied to study the mean difference in albumin and bicarbonate values between acidosis and non-acidosis groups. Receiver operating characteristic (ROC) analysis was done to find the cut-off point of serum tacrolimus level in discriminating acidosis and non-acidosis groups. P value is considered significant at 5% level of significance for all comparisons.
Results
The mean age of the recipients at transplantation was 32.16 ± 8.61 years with 75% of them being male and dialysis vintage was 8 months with mean post-transplant follow-up period being 26.2 ± 14 months. The primary renal disease could not be diagnosed in 75.47% of the study population with IgA nephropathy (IgAN) and focal segmental glomerulosclerosis (FSGS) being the commonest among those identified. All the 106 study patients were on calcineurin inhibitors (CNI)-based triple maintenance immunosuppression [Table 1].
| Parameters (n=106) | Mean±SD |
|---|---|
| Age, mean±SD | 32.16±8.61 |
| Sex, n (%) | |
| Male | 79 (74.53) |
| Female | 27 (25.47) |
| Dialysis vintage, mean±SD | 8 (4, 12)¥ |
| Serum creatinine, mean±SD | 1.26±0.2 |
| eGFR, mean±SD | 63.85±13 |
| Serum bicarbonate, mean±SD | 21.32±2.97 |
| pH, mean±SD | 7.3±0.59 |
| Serum sodium, mean±SD | 136.28±2.76 |
| Serum chloride, mean±SD | 104.3±3.04 |
| Serum potassium, mean±SD | 4.09±0.67 |
| Albumin, mean±SD | 11.95±1.73 |
| Native kidney disease, n (%) | |
| Unknown | 80 (75.47) |
| IgAN | 4 (3.77) |
| RN | 4 (3.77) |
| FSGS | 15 (14.15) |
| MISC | 3 (2.83) |
eGFR: Estimated glomerular filtration rate, SD: Standard deviation, IgAN: IgA nephropathy, FSGS: Focal segmental glomerulosclerosis, MISC: Miscellaneous, RN: Reflux nephropathy, ¥Median (IQR)
Acidosis was diagnosed in 44 of 106 patients (41.5%) with 23 (52.27%) of these patients having severe acidosis. In the acidosis group, 4 patients had high anion gap acidosis, while 40 (90.90%) had RTA. The patients with high anion gap acidosis had a lower GFR ranging between 40 and 44 ml/min/m2. In addition, two of these patients with recently diagnosed post-transplant diabetes mellitus (PTDM) had uncontrolled hyperglycaemia. On further analysis of the RTA group, type I distal RTA was found to be the commonest subtype (n = 23, 52.5%) followed by type IV distal RTA in 12 patients (30.9%). Type II proximal RTA characterised by a urine pH of <5.5 was diagnosed in 3 of 44 acidotic patients [Table 2].
| Parameters | n (%) |
|---|---|
| Acidosis (n=106) | |
| Yes | 44 (41.51) |
| No | 62 (58.49) |
| Severe acidosis | |
| Severe acidosis | 23 (52.27) |
| Acidosis | 21 (47.73) |
| SAG (n=44) | |
| Normal | 40 (90.90) |
| High | 4 (9.09) |
| UAG (n=40) | |
| Positive | 40 (100) |
| Negative | 0 |
| Type of acidosis (n=44) | |
| Type I distal RTA | 25 (52.5) |
| Type II proximal RTA | 5 (7.5) |
| Type IV distal RTA | 12 (30.9) |
| High anion gap | 4 (9.1) |
SAG: Serum anion gap, UAG: Urine anion gap, RTA: Renal tubular acidosis
On comparison of acidosis and non-acidosis groups, serum bicarbonate and eGFR were found to be significantly low in RTA group. Despite low eGFR [odds ratio (OR) – 0.92, P = 0.01] in acidosis group the correlation between eGFR and acidosis was minimally linear (r = 0.1088) implying role of other risk factors for acidosis [Figure 1]. Neither the recipient age and sex nor the donor age and sex was different amongst acidosis and non-acidosis groups. Dialysis vintage and the other peri-transplant factors including type of the graft (living vs cadaver), cold ischaemia time, delayed graft function/slow graft function (DGF/SGF) and nadir creatinine were not different among the two groups neither in the early post-transplant period nor in the long-term. The presence of hypertension and PTDM was not different between the acidosis and non-acidosis groups. Similarly, 13 of 106 patients had proteinuria of 1+ and were equally distributed among the acidosis and non-acidosis group. The presence of previous acute rejection episodes, high-serum tacrolimus levels and to some extent the tacrolimus dosage were found to be significantly high in the RTA groups by univariate analysis. Intake of non-immunosuppressive drugs like cotrimoxazole, angiotensin-converting enzyme/angiotensin II receptor blockers (ACE/ARB) and non-vegetarian (animal protein) food were associated with high risk, while metformin usage for PTDM had no impact on acidosis [Table 3].
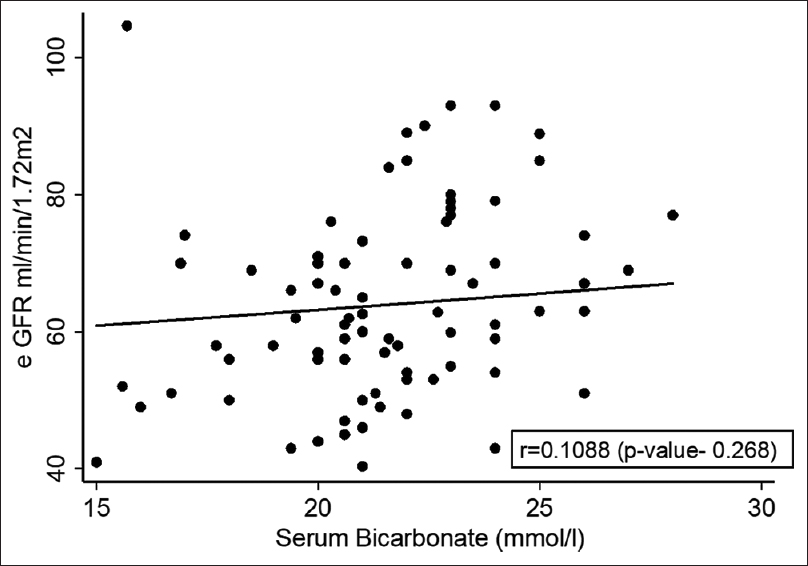
- Estimated glomerular filtration rate and bicarbonate level univariate correlation
| Acidosis | OR | 95% | CI | P |
|---|---|---|---|---|
| Age | 1.00 | 0.96 | 1.05 | 0.918 |
| Sex | 0.67 | 0.27 | 1.64 | 0.378 |
| Duration in HD (months) | 1.00 | 0.97 | 1.03 | 0.902 |
| Donor age | 0.97 | 0.94 | 1.01 | 0.18 |
| Donor sex | 1.16 | 0.53 | 2.52 | 0.706 |
| Induction agent | ||||
| Nil | Reference | |||
| ATG | 1.99 | 0.79 | 5.02 | 0.145 |
| Basiliximab | 0.94 | 0.25 | 3.56 | 0.931 |
| Live vs cadaver | 1.62 | 0.67 | 3.96 | 0.286 |
| CIT (h) | 1.10 | 0.96 | 1.27 | 0.164 |
| DGF/SGF | 1.46 | 0.65 | 3.27 | 0.354 |
| Best creat (mg/dl) | 0.28 | 0.03 | 2.58 | 0.261 |
| Creatinine (mg/dl) | 3.26 | 0.46 | 22.98 | 0.236 |
| eGFR (MDRD) - ml/min/1.73 m2 | 0.96 | 0.93 | 0.99 | 0.02 |
| Duration post-TX (months) | 0.99 | 0.97 | 1.02 | 0.689 |
| Acute rejection | 1.47 | 1.25 | 1.86 | 0.04 |
| Tacrolimus level (last) (ng/ml) | 1.64 | 1.25 | 2.15 | <0.001 |
| Tacrolimus level (mean) (ng/ml) | 1.50 | 1.17 | 1.92 | 0.04 |
| Tacrodose (current) (mg/dl) | 1.25 | 1.00 | 1.55 | 0.051 |
| Metformin | 1.71 | 0.54 | 5.41 | 0.358 |
| Cotrimoxazole | 4.72 | 1.84 | 12.13 | 0.001 |
| ACEI/ARB | 10.54 | 1.31 | 84.95 | 0.027 |
| Food (non-veg) | 4.85 | 2.06 | 11.41 | <0.001 |
HD: Haemodialysis, CIT: Cold ischemia time, DGF: Delayed graft function, SGF: Slow graft function, eGFR: Estimated glomerular filtration rate, MDRD: Modification of Diet in Renal Disease, CI: Confidence interval, OR: Odds ratio, ACEI: Angiotensin-converting enzyme, ARB: Angiotensin II receptor blockers, ATG: Anti - thymocyte globulin
On multivariate analysis, eGFR and history of acute rejection episodes at any time post-transplant was found to be independent risk factors for RTA. The patient's current serum tacrolimus levels were also independently predicting RTA while mean serum tacrolimus levels and current tacrolimus dose were found to be non-significant. The ROC analysis of serum tacrolimus levels in our series showed a level >5.9 ng/ml to predict acidosis with 70% sensitivity and specificity [Figure 2]. Intake of animal proteins and long-term cotrimoxazole prophylaxis were also found to be risk factors for RTA [Table 4]. The serum albumin level was significantly low in the acidotic group correlating linearly with fall in serum bicarbonate levels [Figure 3].

- Receiver operating characteristic of serum tacrolimus levels
| Acidosis | OR | 95% | CI | P |
|---|---|---|---|---|
| eGFR | 0.92 | 0.87 | 0.98 | 0.01 |
| Acute rejection (yes) | 2.00 | 1.21 | 3.32 | <0.001 |
| Tacro level-last (ng/ml) | 1.51 | 1.02 | 2.24 | 0.04 |
| Tacro level-mean (ng/ml) | 1.19 | 0.75 | 1.86 | 0.43 |
| Tacro dose current (mg/dl) | 1.21 | 0.83 | 1.77 | 0.33 |
| Cotrimoxazole | 13.86 | 2.23 | 86.19 | 0.01 |
| ACEI/ARB | 9.92 | 0.74 | 133.15 | 0.08 |
| Food (animal protein) | 6.04 | 1.33 | 27.47 | 0.02 |
eGFR: Estimated glomerular filtration rate, CI: Confidence interval, OR: Odds ratio, ACEI: Angiotensin-converting enzyme, ARB: Angiotensin II receptor blockers

- Metabolic acidosis and albumin levels
Discussion
The prevalence of metabolic acidosis was found to be 41.5% in long-term stable allograft recipients. These numbers are comparable with earlier observations which used similar cut-off of serum bicarbonate of <22 mEq/L. Studies with similar design done by Keven et al. and Malik et al. reported the prevalence to be 33% and 40%, respectively.[47] However, the largest series reported with 823 patients reported by Yakupoglu et al. showed the prevalence to be as high as 58% and that could be attributed to higher cut-off of serum bicarbonate (<24 mEq/L) used in this study.[5] Similarly, Schwarz et al. used a pH cut-off of 7.5 and found the prevalence to be 13%.[3] The variations in the prevalence amongst the literature could also be attributed to variability in transplant eras, time of assessment post-transplant, different cut-offs for graft function in addition to usage of different bicarbonate thresholds.
Most of the above studies have reported type I distal RTA as the most common subtype in the renal transplant recipients with type II and type IV being uncommon.[345] In our series as well, type I RTA was the most common subtype being seen in 53% of these patients. However, type IV RTA was also highly prevalent in our group with a prevalence of 30%, a finding which has differed from the studies of other ethnic population. This might be explained by the effect of CNI, ACE inhibitors (ACEI) and cotrimoxazole on acidosis which was found to be significantly higher in our study, while being variable in other reported literature.
In addition, four patients in our series had high anion gap metabolic acidosis and were evaluated further. All the four patients in this group had a relatively lower eGFR ranging between 40 and 44 ml/min/1.73 m2 and two of these patients had been recently diagnosed with PTDM and uncontrolled hyperglycaemia. However, the aetiology could not be ascertained in the two other recipients with high anion gap acidosis. None of the above mentioned studies report the presence of high anion gap acidosis in transplant recipients.[345]
The renal graft being solitary functioning kidney, the reduced nephron mass would result in generalised defects of acid handling by the renal tubules an effect compounded by low GFR in dysfunctional grafts.[8] The eGFR was found to be lower in the RTA group, an observation similar to that by Keven et al. and Malik et al. and other previous studies.[47] However, this relationship between eGFR and serum bicarbonate was just about linear implying other factors to be involved in the pathogenesis of RTA. Proteinuria of 1+ was noted in 13/106 recipients who had mild graft dysfunction (eGFR between 40 and 60 ml/min/1.73 m2) and was equally distributed amongst the acidosis and non-acidosis groups.
Peri-transplant factors have been advocated as contributors to metabolic acidosis in number of observations. The quality of organ (living vs deceased donor) as well the graft function (prior to and immediately after surgery) had been shown to play a role in acidosis. Keven et al. in his observation of 109 transplant patients reported higher prevalence of acidosis in deceased donor transplants.[4] However, our study did no show such differential effect between donor types. This goes with observations of three other studies by Schwarz et al., Dagan et al. and Kocyigit et al. that looked up the effect of donor source and acidosis.[3910]
The presence of acute rejection (>6 months prior to study) was found to be associated with higher prevalence of RTA, a feature reported by Schwartz and Malik in their study.[37] The higher CNI levels maintained in such immunologically high risk patients might be a contributing factor but multivariate analysis revealed previous rejection episodes to be an independent risk factor. The expression on H+ ATPase in the distal nephron has been found to be decreased by immunohistochemistry staining and direct immunological injury has been postulated as a plausible mechanism in patients with acute rejection.[11] However, the effect of previous rejection episodes on long-term expression of H+ ATPase need to be studied.
The current serum level of tacrolimus (C0) was found to be an independent risk factor for RTA while the mean serum tacrolimus levels and current tacrolimus dosage did not seem to have any effect. This observation of ours stood different from studies by Yakupoglu and Schwartz who found no such association. These authors concluded that better human leukocyte antigen (HLA) match and living donor transplants would have been associated with low levels of CNI exposure.[35] However literature shows higher association of RTA with tacrolimus over cyclosporine similar to the observation by Heering et al.[12] All our patients were on tacrolimus-based immunosuppression and hence class difference in this effect could not be delineated.
The mechanism of CNI-induced functional tubular toxicity has been shown to be calcineurin inhibition independent mechanism. CNIs inhibit the enzyme peptidyl prolyl cis-trans isomerase (PPIase), an enzyme involved in polymerisation of Hensin. This interferes with functioning of cortical intercalated cells involved in H+ secretion.[1314] Moreover, the RTA inducing effects of CNI are dependent on drug dose and reversible especially if the drug is withdrawn or reduced early.[15] However, there is paucity of literature with respect to the tacrolimus dose or levels associated with increased levels of acidosis. The ROC analysis of serum tacrolimus levels in our series showed a level >5.9 ng/ml to predict acidosis with 70% sensitivity and specificity. To our knowledge, this is the first Indian study to determine the serum tacrolimus levels and its correlation with RTA.
Non-immunosuppressive drugs including ACEI/ARB and cotrimoxazole were also found to be associated with RTA. Schwartz et al. made similar association in his study in about 25% of his patients.[3] Literature from the Asian cohort also reports association between ACEI and ARB usage and type IV RTA, an effect that has been attributed to the aldosterone inhibiting effect of RAAS inhibitors.[7] However, this association was moderately significant in our series. Lin et al. reported type I RTA in RTRs recipients which was reversible on cessation of drug intake.[16] This drug has been demonstrated to block the renal tubular sodium channels, reducing hydrogen ions and potassium excretion. In our study we have noted significant association of cotrimoxazole usage in RTA group. This is the first Indian series to show this relationship, an effect that was probably predisposed by unit's protocol to continue cotrimoxazole prophylaxis for life.
In our series, 21 of the 106 recipients developed PTDM and as per the unit protocol were started on metformin as the first line of management. However, the presence of PTDM as well as the use of metformin was not associated with any increased risk for the development of metabolic acidosis in our study similar to observation by Schwartz et al.[3] Similarly, of the 70 patients who were hypertensive before surgery, 56 recipients remained hypertensive after transplant and the presence of hypertension did not have any impact on the development of acidosis. None of the patients in our study population were taking any diuretics thereby removing their confounding effect on serum potassium levels.
The intake of animal protein has been associated with increased acid load which contributes to RTA. Van den Berg et al. demonstrated patients with high intake of animal protein (i.e., from meat, cheese and fish) and low intake of fruits and vegetables had significantly lower serum bicarbonate and serum pH.[17] We did find an association between intake of animal proteins for >3 days in a week and acidosis.
The limitations of our study include sample size and the cross-sectional design. The bicarbonate loading test to assess the proximal tubular dysfunction and measurement of 24 h net acid excretion (NAE) to assess the effect of dietary factors on acidosis could not be done due to logistical issues. The tacrolimus cut-off of 5.9 ng/ml was able to predict acidosis only with 70% sensitivity and specificity thereby undermining diagnostic value.
Despite these shortcomings, our series showed light on the magnitude of metabolic acidosis amongst Indian renal transplant recipients.
Conclusions
In summary, we were able to demonstrate a significant prevalence of distal RTA in RTRs with adequate graft function and large proportion of them being hypoalbuminemic. Screening of RTRs on regular basis is a feasible and cost-effective approach for early diagnosis. Previous acute rejection episodes, current serum tacrolimus trough levels, cotrimoxazole and animal protein intake were found to have significant association with acidosis. However, the weightage for the causal association of each of these factors to acidosis is far from being defined. Further, studies to assess the effect of bicarbonate supplementation on graft survival and metabolic parameters of RTRs will be needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Renal tubular acidosis after cadaver kidney homotransplantation. Studies on mechanism. Am J Med. 1967;42:284-92.
- [Google Scholar]
- KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1-155.
- [Google Scholar]
- Complete renal tubular acidosis late after kidney transplantation. Nephrol Dial Transplant. 2006;21:2615-20.
- [Google Scholar]
- Renal tubular acidosis after kidney transplantation – Incidence, risk factors and clinical implications. Nephrol Dial Transplant. 2007;22:906-10.
- [Google Scholar]
- Posttransplant acidosis and associated disorders of mineral metabolism in patients with a renal graft. Transplantation. 2007;84:1151-7.
- [Google Scholar]
- Metabolic acidosis of CKD: Diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005;45:978-93.
- [Google Scholar]
- Prevalence and risk factors of renal tubular acidosis after kidney transplantation. J Pak Med Assoc. 2011;61:23-7.
- [Google Scholar]
- Posttransplant metabolic acidosis: A neglected factor in renal transplantation? Curr Opin Nephrol Hypertens. 2007;16:379-87.
- [Google Scholar]
- Tubular and glomerular function in children after renal transplantation. Pediatr Transplant. 2005;9:440-4.
- [Google Scholar]
- Renal tubular acidosis in renal transplantation recipients. Ren Fail. 2010;32:687-90.
- [Google Scholar]
- The effect of kidney transplantation on distal tubular vacuolar H+-ATPase. Transplantation. 2008;85:391-7.
- [Google Scholar]
- Cyclosporin A produces distal renal tubular acidosis by blocking peptidyl prolyl cis-trans isomerase activity of cyclophilin. Am J Physiol Renal Physiol. 2005;288:F40-7.
- [Google Scholar]
- Role of hensin in mediating the adaptation of the cortical collecting duct to metabolic acidosis. Curr Opin Nephrol Hypertens. 2005;14:383-8.
- [Google Scholar]
- Hyperchloremic metabolic acidosis with high serum potassium in renal transplant recipients: A cyclosporine A associated side effect. Clin Nephrol. 1986;25:245-8.
- [Google Scholar]
- Reversible voltage-dependent distal renal tubular acidosis in a patient receiving standard doses of trimethoprim-sulphamethoxazole. Nephrol Dial Transplant. 1997;12:1031-3.
- [Google Scholar]
- Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol. 2012;7:1811-8.
- [Google Scholar]







