Translate this page into:
Community Acquired AKI: A Prospective Observational Study from a Tertiary Level Hospital in Southern India
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Pattern of acute kidney injury (AKI) differs vastly from region to region in India. Moreover, prospective data on community-acquired AKI (CAAKI) using the KDIGO criteria for AKI are limited. Our objective was to study the etiology, clinical characteristics, and short-term outcome of CAAKI in adults.
Methods:
This was a prospective observational study in the medical wards of a tertiary care hospital. Patients fulfilling the 2012 KDIGO AKI criteria of community acquired acute kidney injury (CAAKI) were included. Patients who developed AKI 48 hours after admission, those hospitalized >48 hours elsewhere, and patients with chronic kidney disease were excluded. The study did not include obstetric or surgical cases of AKI. Serum creatinine and urine output was monitored. Daily progress, in particular development of hypotension, oliguria, acute respiratory distress syndrome, sepsis, and renal replacement therapy, was noted.
Results:
Of 186 CAAKI patients (mean age, 46.13 ± 15.2 years), 86/186 was infective etiology, 93/186 was non-infective etiology, 7/186 was due to intrinsic renal pathology. Pyelonephritis 33/186 (17.7%) was the most common infective etiology, and snakebite in 49 (26.3%) was the most common non-infective etiology; 28/186 (15.1%) died. On logistic regression, hypotension, mechanical ventilation, thrombocytopenia, and anuria were associated with mortality.
Conclusions:
Acute pyelonephritis and snakebite-related AKI emerged as the two most common medical causes of CAAKI in our region. Such environmental and infectious causes that largely are preventable causes of AKI are also associated with significant morbidity and mortality.
Keywords
Acute kidney injury
acute kidney injury
community-acquired AKI community-acquired AKI
dialysis
Introduction
Hospital-acquired AKI (HAAKI) are typically drug-induced, sepsis-related, post contrast administration, post-surgical, and caused by hemorrhage.[1] While HAAKI forms the bulk of AKI in developed nations, in tropical countries like India, AKI is mostly community acquired (CAAKI), i.e., occurs outside the hospital setting, the causes of which are dehydration, diarrhea, infections, and venomous snakebite, etc.[2] The demographics and etiology of CAAKI is known to vary among countries in Asia, with patients from India being much younger than those in China. Moreover, within the Indian subcontinent, there is variation in the etiology of CAAKI reported between centers, which are geographically distant, and the etiologic spectrum has been demonstrated to change over passage of time. For example, in the study by Prakash et al. from the eastern part of India comparing AKI between 1983–1995 and 1996–2008, it was found that the incidences of obstetrical, surgical, and diarrheal AKI decreased significantly, whereas AKI associated with malaria, sepsis, nephrotoxic drugs, and liver disease increased.[3] The incidence of renal cortical necrosis also reduced significantly.[4] From the northern part of the country, in the study from Chandigarh involving 1862 patients over a period of 21 years (1965–1986), the bulk of AKI cases were medical causes (60%), whereas obstetric and surgical constituted 15% and 25% causes of AKI, respectively.[5] Diarrheal diseases-related AKI drastically reduced, whereas drug-related and sepsis-related AKI increased. Similarly, obstetric causes of AKI reduced. These studies also highlighted that the pattern of AKI in India is different from industrialized nations in that there is a significant component of CAAKI. While the etiologic spectrum of AKI in northern India is well documented, there is limited literature on the etiology and outcomes of CAAKI from southern India. A study from Chennai reported diarrhea as the most common cause of AKI, followed by drugs, glomerulonephritis, sepsis, snakebite, leptospirosis, malaria, and copper sulfate as other common causes.[6] The spectrum of AKI was different from reports from northern Indian states.
AKI has been the focus of extensive clinical and basic research efforts over the last decades.[7] Definitions for AKI have largely varied and majority of published studies on AKI have been retrospective in design. The Kidney Disease: Improving Global Outcomes (KDIGO) definition for AKI published in 2012 is the result of the collaborative efforts of nephrologist and critical care specialist society designed to diagnose AKI very early so that patient outcomes can be improved.[8]
Prospective data from India on CAAKI using the new definition are limited. The objective of the study was to study the etiology, clinical characteristics, and short-term outcome of CAAKI patients admitted in the medical wards.
Materials and Methods
Setting and design
We conducted a prospective, cross-sectional study from September 2013 to June 2015 at the Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) hospital, which is a tertiary care hospital located in southern India. The catchment area of the hospital includes towns and villages of Villupuram, Cuddalore, Thiruvannamalai, Kanchipuram, as well as other districts of Tamil Nadu state (79%) and Puducherry (19%). Adult patients admitted in medicine through the emergency fulfilling the 2012 KDIGO criteria for AKI were included if AKI was present at admission or it developed within 48 hours of hospital admission. Hence, we defined CAAKI as the presence of AKI at admission or developing AKI within 48 hours of hospital admission. Patients who developed AKI beyond 48 hours of admission stay, as well as those who have been hospitalized elsewhere for a period of more than 48 hours were excluded. This was done to exclude hospital-acquired AKI. Patients with chronic kidney disease (evidence of structural damage of kidneys by ultrasonography or history of renal insufficiency more than 3 months) were also excluded.
The sample size was calculated based on the formula to estimate a single proportion. With an expected mortality of 22%[9] at 90% confidence level and relative precision of 5%, the required sample size was 186. Written informed consent was obtained from all study participants before enrolling them in the study. The study protocol was approved by the institute ethics committee.
Study procedure
Patients were admitted through the emergency to medical wards and medicine ICU. Detailed history and clinical examination were performed, records were checked to confirm the presence of comorbid conditions namely diabetes, hypertension, HIV or HBsAg positivity, or HCV positivity, among others. Serum creatinine at admission, baseline serum sodium, serum potassium, liver functions, hemoglobin, total leukocyte count, and platelet count was done. During hospital stay, the patient was followed up with daily clinical examination. Urine output was measured every 6 hours on day 1 of hospital stay and every 24 hours later on for patients in the general ward. For patients in the ICU, urine output was measured every 6 hours. Development of hypotension, oliguria, acute lung injury (ALI), acute respiratory distress syndrome (ARDS), encephalopathy, sepsis, and thrombocytopenia, requirement for mechanical ventilation and the duration of ventilation were noted. Workup to achieve a definitive diagnosis was individualized for each patient. Repeat tests for serum creatinine were performed serially and were individualized for each patient. The maximum stage of AKI and maximum serum creatinine for each patient was noted. Management of patient such as antibiotic use, enteral nutrition protocol, fluids administered, vasopressors, management of hypoglycemia, decision on dialysis, and administration of blood products were determined by individual unit's policy. Requirement of dialysis, i.e. renal replacement therapy (RRT), was initiated in consultation with nephrologist. The type of dialysis (hemodialysis, slow low efficiency dialysis), and the total number of dialysis sessions required were individualized.
Each patient was followed up till discharge or death. Complete renal recovery was defined as return of serum creatinine to <1.5 mg/dL and nonoliguric state. Partial renal recovery was defined when serum creatinine at discharge was declining but did not return to normal. When serum creatinine was persistently high at discharge or death, it was assumed that renal recovery did not occur.
Definitions
Hypotension was defined as systolic BP <90 mmHg or requirement of inotropes to maintain a mean arterial pressure (MAP) of 65 mmHg. Oliguria was defined as urine output <0.5 ml/kg/hour for more than 6 hours. Anuria was defined as urine output <100 ml for 12 hours. ARDS was defined by the Berlin definition for ARDS.[10] Encephalopathy was defined as any decline in mental status, asterixis, or other neurologic symptoms in the concurrent presence of high urea and creatinine, provided there was no intracranial pathology or liver cirrhosis. Thrombocytopenia and anemia were defined as platelet count <100,000/dL and hemoglobin (Hb) <9 g/dL, respectively. Sepsis was defined as per the Surviving Sepsis Campaign definition.[11] Hypertension was defined as systolic BP >150 mmHg and/or diastolic BP >90 mmHg. Hyponatremia and hypernatremia was defined as serum sodium level <135 meq/L and >145 meq/L, respectively. Hypokalemia and hyperkalemia was defined as serum potassium level <3.5 meq/L and >5.5 meq/L, respectively.
Method of statistical analysis
Categorical data related to clinical characteristics (hypotension, hypertension, oliguria, acute lung injury/ARDS, encephalopathy, and thrombocytopenia), final diagnosis, outcome, requirement of dialysis, and ventilator requirement were presented as frequencies/percentages. Continuous variables such as age, duration of illness, number of days in hospital, number of days on ventilator, biochemical parameters (Hb, TLC, platelets, urea, creatinine) were presented as mean ± SD. Non-normal data were presented as median with range. The comparison of survivors or nonsurvivors were carried out by independent Student's t-test/Mann–Whitney U-test, whichever was appropriate. Logistic regression was used to identify the individual factors associated with outcome. All statistical analysis was carried out with 5% significance, and P value < 0.05 was considered significant.
Results
One hundred and eighty-six patients of CAAKI were studied between September 2013 and June 2015. The mean age of the patients was 46.13 ± 15.2 years. Out of 186, 74 patients (40%) were aged ≤40 years, 82 (44%) were 41–60 years, and 30 (16%) were ≥61 years. Majority of patients were males n = 150 (80.6%). The baseline characteristics are shown in Table 1.
| Clinical characteristic | CAAKI, n=186 |
|---|---|
| Age in years (mean±SD) | 46±15.2 |
| Diabetes, n (%) | 32 (17) |
| History of Hypertension, n (%) | 3 (1.6) |
| HBsAg positive individuals, n (%) | 3 (1.6) |
| HIV, n (%) | 3 (1.6) |
| Duration of illness in days, median, IQR | 3 (1-7) |
| Hypotension, n (%) | 21 (11.3) |
| Oliguria, n (%) | 67 (36) |
| Anuria, n (%) | 7 (3.8) |
| Hypertension at admission, n (%) | 19 (10.2) |
| Baseline laboratory parameters | |
| Serum creatinine (mg/dL), median IQR | 2.3 (1.5-4.07) |
| Patients with serum creatinine <1.2 mg/dL at baseline, n (%) | 29 (15.6) |
| Hemoglobin (g/dL), mean±SD | 10.8±2.8 |
| Total leukocyte count/µL median (range) | 13200 (9025-19775) |
| Platelet counts/µL median (range) | 186×103 (87×103-255×103) |
| Patients with anemia, n (%) | 31 (16.7) |
| Patients with thrombocytopenia, n (%) | 50 (27) |
| Hyponatremia, n (%) | 90 (48) |
| Hyperkalemia, n (%) | 19 (10) |
Fifty-five (29.6%) patients developed hypotension during the hospital stay. Oliguria was observed in 50% of patients during the hospital stay. Encephalopathy was seen in 19 (10.2%) patients. Twenty-four (12.9%) patients had sepsis, whereas ARDS was seen in 34 (18.27%); mild ARDS in 31 (16.7%); and moderate ARDS in 3 (1.6%) patients. During the course of the hospital stay, 40 patients (21.5%) required mechanical ventilation. The median duration of ventilation was 5 days, ranging from 1 to 35 days.
Stage of AKI and renal replacement therapy
Out of the 186 patients, 70 (37.6%) were AKI stage 1, 46 (24.7%) AKI stage 2, and 70 (37.6%) AKI stage 3. This was the maximum AKI stage reached during the entire course of hospital stay. The median creatinine reached by patients with AKI was 2.8 mg/dL (range, 1.2 to 25.3). Forty of 186 patients (21.5%) required renal replacement therapy (RRT). The predominant form of RRT was intermittent hemodialysis received by 31/40 (77.5%) patients, while 9/40 (22.5%) patients who had hypotension received sustained low efficiency dialysis (SLED). The median number of dialysis sessions received was 3 (range 1 to 10).
Etiology of CAAKI
In 86 of 186 patients (46.23%) the underlying cause for AKI was infective; 93/186 (50%) had a non-infective underlying clinical condition; and remaining 7/186 (3.8%) had intrinsic renal pathology, namely post-infectious glomerulonephritis in 4/186 (2.2%) and acute interstitial nephritis in 3/186 (1.6%) patients. Of the patients with infective cause, pyelonephritis was the most common infection seen in 33/186 (17.7%), followed by pneumonia in 10/186 patients (5.4%). Ten of the 33 (30%) patients with pyelonephritis were diabetics (P = 0.04). The other infective conditions are shown in Figure 1. Among the non-infective conditions of CAAKI, the most common cause was snake bite in 49 patients (26.3%). The other non-infective conditions are depicted in Figure 1.
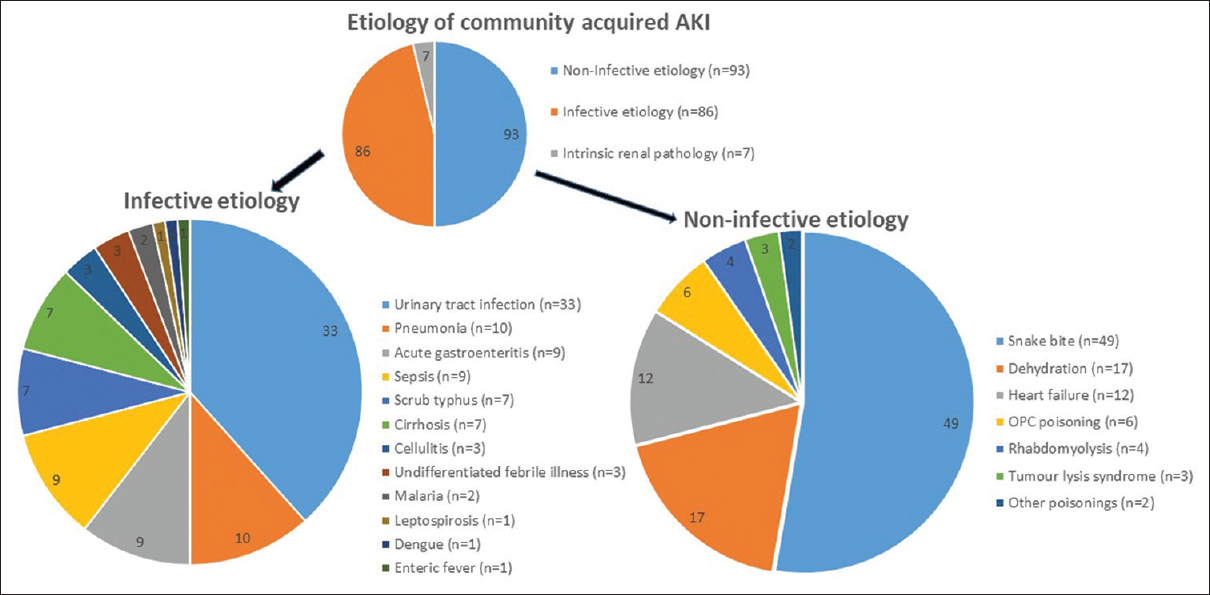
- Infective and noninfective etiology associated with community-acquired acute kidney injury (CAAKI). Urinary tract infection (pyelonephritis) was the major infective cause whereas snakebite-related AKI was the major noninfective cause. Sepsis included sepsis due to liver abscess, endopthalmitis, pemphigus vulgaris, and other cases, where source of sepsis was not identified, whereas patients with urospesis were not included in this subheading. Other poisonings included one patient with paraquat and one with hair dye poisoning
Outcome and renal recovery
Twenty-eight of 186 patients (15.1%) died, of which 9 (4.8%) patients died within 72 hours. Among 28 patients who died, 9 were between 18 and 40 years, 17 were between 41 and 60 years, and 2 were ≥60 years. Of the 28 deaths, 7 (25%) were snake bite related AKI, 5 (17.8%) were patients with pyelonephritis, 3 (10.7%) were organophosphorus compound poisoning, 4 (14.3%) were patients with community acquired sepsis, 2 (7%) patients had moderate ARDS, and 2 (7%) were AKI related to heart failure. The remaining 5 who died included one patient each with interstitial nephritis, leptospirosis, enteric fever, stroke with dehydration, and acute disseminated encephalomyelitis. The cause of death in a majority of the patients was multiorgan failure and hospital-acquired infections. The median duration of hospital stay in the entire study group was 9 days (range, 2-55 days). Among the 158 survivors, 71 (45%) had complete renal recovery, 75 (47.4%) had partial renal recovery, whereas 12 (7.6%) did not have renal recovery at discharge.
Comparison of clinical features between survivors and non-survivors
Presence of hypotension, oliguria, anuria, ARDS, encephalopathy, mechanical ventilation, and sepsis was significantly associated with mortality [Table 2]. However, on logistic regression analysis only the presence of hypotension, mechanical ventilation, thrombocytopenia, and anuria were significantly associated with adverse outcome, i.e., death. There was no significant difference between AKI stages and outcome.
| Clinical feature | Survivor (n=158) | Nonsurvivor (n=28) | Statistical significance (P) |
|---|---|---|---|
| Male gender, n (%) | 131 (87.3) | 19 (12.7) | 0.063 |
| Female gender, n (%) | 27 (75) | 9 (25) | |
| Age in years, mean±SD | 46.2±15.6 | 45.7±13.2 | 0.887 |
| Presence of comorbid conditions, n (%) | 40 (25.3) | 4 (14.3) | 0.206 |
| Serum creatinine mg/dL, mean±SD | 3.45±3.1 | 3.25±3.4 | 0.760 |
| Maximum serum creatinine, mean±SD | 4.23±3.75 | 5.43±3.95 | 0.127 |
| Hemoglobin, g/dL mean±SD | 10.8±2.73 | 11.11±3.24 | 0.651 |
| Hypotension, n (%) | 34 (21.5) | 21 (75) | <0.001* |
| Oliguria, n (%) | 72 (45.5) | 21 (75) | 0.004* |
| Anuria, n (%) | 2 (1.2) | 5 (17.8) | <0.001* |
| Lung involvement (ARDS), n (%) | 24 (15.2) | 12 (42.8) | 0.001* |
| Encephalopathy, n (%) | 13 (8.2) | 6 (21.4) | 0.035* |
| Sepsis, n (%) | 14 (8.8) | 10 (35.7) | <0.001* |
| Hyponatremia, n (%) | 79 (50) | 11 (39.3) | 0.307 |
| Hypernatremia, n (%) | 5 (3.1) | 2 (7.1) | 0.284 |
| Hyperkalemia, n (%) | 16 (10.1) | 3 (10.7) | 0.925 |
| Anemia, n (%) | 41 (26) | 8 (28.5) | 0.772 |
| Thrombocytopenia, n (%) | 52 (33) | 16 (57) | 0.014* |
| Ventilator requirement, n (%) | 15 (9.4) | 25 (89.3) | <0.001* |
| RRT, n (%) | 31 (19.6) | 9 (32) | 0.137 |
| Infectious etiology, n (%) | 75 (47.4) | 11 (39.2) | 0.710 |
| Initial abnormal serum creatinine, n (%) | 136 (86) | 21 (75) | 0.136 |
| AKI stage 1, n=70 | 63 (90) | 7 (10) | 0.069 |
| AKI stage 2, n=46 | 41 (89.1) | 5 (10.9) | |
| AKI stage 3, n=70 | 54 (77.1) | 16 (22.9) |
CAAKI: Community-acquired acute kidney injury, ARDS: Acute respiratory distress syndrome, Anemia defined as hemoglobin <9 g/dL, thrombocytopenia defined as platelet count <100,000/dL; RRT: Renal replacement therapy
The mean duration of hospital stay was 9 days in patients with AKI stage 1, 12.5 days in AKI stage 2, and 14 days in AKI stage 3 (P = 0.003). As expected, majority of patients (49/70, 70%) with stage 1 AKI had complete renal recovery in contrast to stage 3 AKI patients where only 6/70 (8.6%) had complete renal recovery (P < 0.001).
Discussion
We prospectively studied 186 patients with CAAKI over 17 months. An important observation which emerges from our study is that the most common cause of CAAKI was snakebite, which was seen in 49 of 186 (26%) cases. In previous Indian studies, AKI caused by snakebite was comparatively less. In the study by Muthusethupathi et al. in Chennai,[12] only 6 out of 187 patients had AKI due to snake bite. In a more recent study from Chennai, only 4.7% of AKI cases were due to snakebite.[6] In a study on CAAKI from Himachal Pradesh,[13] only 4 out of 102 patients had AKI due to snakebite, and in the latest study from Vishakhapatnam,[14] snakebite causing AKI was found to be present in only 7.5% of all cases. However, a direct comparison with these studies is not appropriate because we recruited only CAAKI from medical causes, whereas other studies included medical, obstetric, and surgical AKI and both CAAKI and HAAKI. In the study from Lucknow, which studied only CAAKI, of the 186 medical causes of CAAKI, there were no snakebite-related AKI.[9] The high percentage of snakebite-related AKI in our study is probably because our hospital caters to districts where agriculture is the main occupation, and our patient population comprise largely underprivileged agricultural laborers. There are geographical differences in snakes as well, which might have contributed to this difference, and being a tertiary care center, there is a referral bias as well, where severe snakebite cases were referred.
The second major observation is that pyelonephritis was the second most common cause of CAAKI in 33 of 186 (17.7%) patients. As a policy for all patients suspected to have acute pyelonephritis, a urine sample is collected for culture and sensitivity testing before any antibiotic is started. In the 2011 study from Yemen, only 0.5% of patients with AKI was due to pyelonephritis.[15] Even among Indian studies, few have highlighted pyelonephritis as an important cause of CAAKI. Dhanapriya et al. from Chennai in their analysis of 121 pyelonephritis patients admitted over 22 months, 42.9% were AKI stage 3, and 67% were diabetics.[16] However, in our data of 33 patients with pyelonephritis, only 10 (30%) were diabetic. In patients where urine culture was positive, E. coli was the predominant organism grown showing sensitivity pattern to amikacin, cefoperazone, and meropenem. It appears that acute pyelonephritis is emerging as an important cause of CAAKI in our region, afflicting even those patients without a predisposing factor like diabetes. Regarding our finding of higher frequency of pyelonephritis in the nondiabetics, we feel this may be due to bias in data collection, as we utilized convenient sampling. A better method would be to conduct consecutive sampling to see the true incidence of AKI in diabetic and nondiabetic pyelonephritis patients. Nevertheless, it is disconcerting that multidrug resistant bacteria are the predominant organisms, possibly resulting from rampant and indiscriminate use of antibiotics in the community.
In our study, sepsis was present in 24 (12.9%) patients. Overall, in India and other low-income countries, the percentage of CAAKI due to sepsis is low compared to affluent countries. For example, in the study on CAAKI by Kaul et al. from Lucknow, 26 out of 240 (10%) had sepsis;[9] in the study from Himachal Pradesh (both CAAKI and HAAKI) 32% had sepsis.[13] In a study from Yemen on CAAKI, only 6.9% had sepsis.[15] In contrast, in a study from Spain (both CAAKI and HAAKI) sepsis constituted almost 50% of AKI cases.[17] However, more recent data from Chennai show that sepsis is emerging as the most common medical cause of AKI.[16] In our study, 10 of 24 patients with sepsis died. Patients with sepsis had significantly higher mortality than patients without sepsis as has been documented by other authors.[1218]
Prerenal azotemia has been the most common cause of AKI in many older studies from Chennai,[612] Chandigarh (1989),[5] and Boston (1991).[19] In India, AKI due to acute diarrheal disease ranged from 20.6% to 30.5%.[8111213] In our study, AKI due to acute gastroenteritis was seen in only 4.8% of the patients probably because acute diarrheal disease has been decreasing over the past few years due to better hygiene, better facilities, and effective management in primary and secondary level hospitals.[3] Almost 80% of patients in our study were males, similar to that reported in other studies.[9131520] The higher percentage of males can be due to occupational hazard among males that predisposes them to vector-borne diseases and envenomation.[1]
The mortality among CAAKI patients recorded in our study was 15.1%, with snakebite-related AKI (25%) and acute pyelonephritis (17%) contributing the most. None of our patients with rickettsial infections died. It is difficult to compare our results with other studies because of the heterogeneity of the study groups. Nevertheless, the mortality reported in other studies of CAAKI has been variable, i.e., 26.2%, 6.9%, and 22% from Lucknow,[9] Vishakhapatnam,[14] and Kashmir,[21] respectively, whereas it was 32.7% in a nationwide community-based analysis of AKI cases in the UK.[22] The wide variations in mortality observed is because of different definitions for AKI.[9142122] Because we used KDIGO criteria, patients with even “mild” renal impairment included in the study, most recovered, resulting in overall lower mortality.
Almost 60% of those who died were between 40 and 60 years of age, a point of clinical significance as these patients belong to the economically productive group. It is also well known that older people with AKI have higher mortality.[2223] The predictors of mortality in CAAKI in our study (as has already been shown in previous studies) was presence of hypotension, mechanical ventilation, thrombocytopenia, and anuria.[39131924] There was no relationship between higher admission creatinine and survival in our study, nor with AKI stage; though one study of AKI among patients with tropical febrile illness[23] showed that higher baseline creatinine was associated with mortality.
Only 22% patients required RRT for AKI. RRT requirement in previous studies on CAAKI have been variable, ranging from 7.8% (UK) to 36% (Spain) to 53% (Himachal Pradesh) to 83% (Lucknow).[9131721] In the study from Lucknow on CAAKI, RRT requirement was 83%, most likely because the study setting was a specialized nephrology unit, where more severe cases of AKI were referred. The different RRT requirement rates are also probably due to the different inclusion criteria used. Studies done in nephrology units have always shown higher percentages of RRT requirement.
Complete renal recovery and partial renal recovery was seen in 76 (40.9%) and 79 (42.5%) patients, respectively. Our results are comparable to the study by Kaul et al.,[8] where 44% had complete recovery, whereas 13% had partial recovery. However, in the study by Kumar et al.,[13] complete recovery was seen in almost 70%.
Limitations of this study are that the causes of CAAKI are limited to medical causes, whereas obstructive and obstetric causes were not included. Second, we did not find patients diagnosed with primary glomerular disease. This is in contrast to the study from Lucknow where patients were diagnosed with microangiopathy also.[9] Third, patients were followed up only till discharge; no further follow-up was done. Hence, data on recovery of patients is not complete. Strengths are that our study is a prospective study using KDIGO criteria. Patients with oliguria were also included according to the urine output definition of AKI.
To conclude, acute pyelonephritis and snakebite-related AKI emerged as the two most common medical causes of CAAKI in our region. Such environmental and infectious causes that are largely preventable causes of AKI are also associated with significant morbidity and mortality. Longitudinal studies with longer follow-up of CAAKI survivors are required, so that measures can be taken to prevent CKD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Community acquired acute kidney Injury in tropical countries. Nat Rev Nephrol. 2013;9:278-90.
- [Google Scholar]
- Changing epidemiology of community-acquired acute kidney injury in developing countries: Analysis of 2405 cases in 26 years from eastern India. Clin Kidney J. 2013;6:150-5.
- [Google Scholar]
- Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: A single-centre experience of 22 years from Eastern India. Nephrol Dial Transplant. 2007;22:1213-7.
- [Google Scholar]
- Changing trends in acute renal failure in third-world countries--Chandigarh study. Q J Med. 1989;73:1117-23.
- [Google Scholar]
- Epidemiologic trend changes in acute renal failure-a tertiary center experience from South India. Ren Fail. 2006;28:405-10.
- [Google Scholar]
- and the Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the Acute Dialysis Quality Initiative Group. Crit Care. 2004;8:R204-12.
- [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO). Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:1-138.
- [Google Scholar]
- Spectrum of community-acquired acute kidney injury in India: A retrospective study. Saudi J Kidney Dis Transpl. 2012;23:619-28.
- [Google Scholar]
- Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012;307:2526-33.
- [Google Scholar]
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-74.
- [Google Scholar]
- Acute renal failure in south India. Our experience with 187 patients. J Assoc Physicians India. 1987;35:504-7.
- [Google Scholar]
- Spectrum of acute kidney injury in the Himalayan region. Indian J Nephrol. 2012;22:363-6.
- [Google Scholar]
- Epidemiology and outcomes of acute renal failure in rural females of low socioeconomic status – A referral hospital experience. Int J Sci Res 2013. [Internet]. Available from: https://www.ijsr.net/archive/v4i1/SUB151027.pdf
- [Google Scholar]
- Acute renal failure in Yemeni patients. Saudi J Kidney Dis Transpl. 2011;22:829-33.
- [Google Scholar]
- Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811-8.
- [Google Scholar]
- Predictors of mortality in acute renal failure in a developing country: A prospective study. Ren Fail. 2007;29:463-9.
- [Google Scholar]
- Differences in community, hospital and intensive care unit-acquired acute kidney injury: Observational study in a nephrology service of a developing country. Clin Nephrol. 2012;78:449-55.
- [Google Scholar]
- Community-acquired acute kidney injury in a tertiary care hospital: A cross sectional study. Int J Sci Stud. 2015;3:58-61.
- [Google Scholar]
- Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292-8.
- [Google Scholar]
- Acute kidney injury in tropical acute febrile illness in a tertiary care centre--RIFLE criteria validation. Nephrol Dial Transplant. 2011;26:524-31.
- [Google Scholar]
- Spectrum of hospital-acquired acute renal failure in the developing countries— Chandigarh study. Q J Med. 1992;83:497-505.
- [Google Scholar]







