Translate this page into:
The Role of Far Infrared Therapy in the Unassisted Maturation of Arterio-venous Fistula in Patients with Chronic Kidney Disease
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
The goal of arterio-venous fistula (AVF) creation is to achieve a well-functioning access that can be cannulated repetitively and can provide adequate flow for the dialysis. The objective of this study was to assess the role of far infrared (FIR) therapy in the unassisted maturation of newly created AVF in patients with chronic kidney disease (CKD).
Materials and Methods:
In this prospective open labeled randomised control trial, 107 patients were randomized. Participants in the control arm received oral clopidogrel 75 mg once daily for 30 days along with isometric hand exercise, whereas those in the test arm received FIR therapy twice weekly, 40 min session each, for 4 weeks. A biopsy from venous end was taken during fistula surgery. Doppler study of AVF was done at the end of the 4th and 12th week to assess AVF. Vascular access guidelines proposed by National Kidney Foundation –Kidney Disease Outcomes Quality Initiative (NKF- KDOQI) in 2006 were adapted to define the maturation of AVF.
Results:
Out of 107 patients, 51 were randomized to the test arm and 56 to the control arm. During follow-up, the blood flow rate through AVF (Qa) and the diameter of the cephalic vein draining (CVd) the AVF were measured. At the end of 3 months, Qa in Radio-Cephalic Fistula (RCF) was high in the test arm (p–0.003). The AVF failures were 5 (10.2%) and 14 (28%) in the test and control arms, respectively (p: 0.025). However, when adjusted for AVF failure within 6 h of surgery (may be related to surgical technique) this difference in AVF patency was statistically insignificant (p: 0.121). The mean Qa was high in patients with an arterial intimal medial thickness (AIMT) <0.5 mm. The IMT of the anastomosed artery had statistically significant correlation with the primary failure rate of AVF (P < 0.001).
Conclusion:
In patients with CKD, FIR therapy was effective in increasing the AVF blood flow rate at the end of 3 months, though the difference in primary failure rate was statistically insignificant.
Keywords
AV fistula
chronic kidney disease
far infrared therapy
far infrared
primary failure
Introduction
A well functioning vascular access is the pre-requisite for successful, long term, and adequate hemodialysis in a patient with chronic kidney disease (CKD) and is therefore essential for improving their quality of life. Among different options for vascular access, native arterio-venous fistula (AVF) is recommended as the best available vascular access by the National- Kidney Foundation- Kidney Diseases Outcomes Quality Initiative (NKF- KDOQI) vascular access guidelines in 2006.[1] The cephalic vein is considered as the best conduit for creating native AVF in the upper limb. Major issue in AVF is its malfunction, which occurs in about 20 to 60% of cases.[2345]
Maturation of newly created AVF is complex. The AVF created should be a circuit with least resistance, so that it can dilate to accommodate the high blood flow needed for hemodialysis. Both the arterial and the venous limbs of a newly created AVF are subjected to significant hemodynamic changes, that stimulate vascular remodeling, which end up in thickening of the vessel wall and increase in the caliber of the vessels. This complex process is known as AVF maturation. These changes are essential for repeated cannulation and for accommodating the cannula inserted. This maturation can be assessed by parameters such as blood flow through AVF, diameter of the arterial and venous limbs of AVF, and its wall thickness.[6]
Although maturation failure is high, the complication profile such as infection and hospitalization rate are very less in matured AVF when compared with the other forms of vascular access such as tunneled catheter and AV grafts.[78] It is also well-documented in the literature that the incidence of stenosis and thrombosis is reduced once the AVF matures and this gets translated into prolonged AVF patency rates.[9]
Numerous factors are known to affect the maturation of an AVF. The important factors that are associated with poor maturation of AVF include poor surgical skill, pre-existing intimal hyperplasia, obesity, diabetes, smoking, longer duration of CKD, AVF infection, and certain genotypic polymorphisms in TGFβ1, hemoxygenase-1, and Methylene tetrahydrofolate reductase (MTHFR). Various factors are also shown to promote the maturation of the native AVF and the blood flow to the fistula. Numerous drugs/non-invasive techniques were tried to accelerate its maturation and to maintain its patency rate.
Earlier studies that were conducted to identify the role of clopidogrel and other antiplatelet agents in the maturation of AVF showed that though these agents reduce the frequency of early thrombosis, they had no repercussion on the cumulative patency (defined as the time from AVF creation to the initial episode of fistula dysfunction).[1011] One such non-invasive and convenient factor known to enhance blood flow and patency rate of AVF is far infrared (FIR) therapy. Animal studies have shown that FIR exposure improves the blood flow to the skin.[121314] This study prospectively analyzed the role of FIR therapy in the maturation of AVF and its role in the maintenance of its patency.
Materials and Methods
Objectives
Primary: To assess the role of FIR therapy in the unassisted maturation of newly created AVF in patients with CKD.
Secondary: To assess the impact of the arterial and venous intimal medial thickness (IMT) on the AVF maturation.
Design of study
The study was a prospective-open labeled randomized control trial conducted in our hospital. This study was according to the Helsinki declaration (edition 6, revised 2000)[15] and was approved by the Institutional ethical committee.
Sample size
According to the study published by Lin et al. in 2008,[16] the malfunction rate of the newly created AVF was 20% higher than that of the prevalent AVF, which were 30% and 12.5% for the control and intervention arms, respectively. On the basis of thesis, the malfunction rate of newly created AVFs was assumed as 36% and 15% for the control and test arms, respectively. The number of patients completing the study should be at least 50 in each arm allowing 10% alpha error to achieve 80% power.
Patient selection
The patients who met the following criteria were included
-
1)
CKD patients with estimated GFR (eGFR) <30 ml/min/1.73 m2, planned for primary AVF [Brachio-cephalic/Radio-cephalic (both proximal and distal)]
-
2)
Age >13 years and <80 years.
The patients who met one or more among the following criteria were excluded from the study
-
1)
Complex vascular access procedures: Re-do brachio-cephalic and radio-cephalic fistulas (RCFs)/Brachio- basilic fistulas (BBFs)/Prosthetic grafts
-
2)
Patients with infections/sepsis within past 2 weeks/History of antibiotic intake within past 1 week
-
3)
Patients with thrombotic tendencies such as lupus anticoagulant/on patients on chronic anti-coagulants
-
4)
Previous history of large vessel thrombosis
-
5)
Cardiovascular dysfunction
Hypotension (systolic <90 mm of hg)
Confirmed myocardial infarction within the last 6 months
LV dysfunction with EF <35%
Obstructive cardiomyopathy
Severe aortic stenosis (gradient >40 mmhg)
-
6)
Marked anemia (Hb <6 gm %)
-
7)
History of hypothyroid disease.
Method
After getting informed consent from each participant, the basic details of the patients and their clinical history were recorded. The duration of CKD, presence of diabetes/hypertension or other comorbidities, and the treatment they pursue for these ailments were noted. At the time of recruitment, all patients were subjected to blood investigations- complete blood count, blood urea, serum creatinine, serum electrolytes, uric acid, calcium, phosphorus, and serum albumin. Their recent ultrasonography for renal size was noted. eGFR was calculated using CKD-EPI equation using recent serum creatinine value for those who were not on hemodialysis and by using creatinine at the time of hemodialysis initiation for the patients on hemodialysis. If patients were on hemodialysis, the dialysis vintage, the duration of each HD, and the complications during HD were noted.
Further, the patients were examined by experienced senior vascular surgeons, working in the department of vascular surgery in our hospital, to decide about the site of AVF creation according to the preliminary ultrasonography of the upper limb vessels. The radial artery IMT and the brachial artery IMT were also noted in all patients. The site of the fistula to be constructed was decided according to vascular mapping. The minimal diameter required for the artery and vein to be considered for the procedure were 2 mm and 2.5 mm, respectively. There should be no stenosis or thrombosis in the draining vein.
Then, the patients were randomly assigned to either of the 2 groups using computerized minimization program to minimize imbalance between the 2 groups. They were randomized only if they were about to undergo initial upper limb AVF surgeries such as RCF and brachio-cephalic fistula (BCF).
Test arm
Patients received FIR therapy 24 h prior to the proposed time of AV fistula creation and FIR therapy twice a week, with each session lasting for 40 min, for 4 weeks. The distance between the source of FIR and the skin was 30 cm, to prevent injury to the skin. Apart from that, the patients of this arm also received oral clopidogrel 75 mg once daily for 30 days along with isometric hand exercise.
Control arm
Patients received oral clopidogrel 75 mg once daily for 30 days along with isometric hand exercise.
Outcome
Physiologic maturation of AVF-defined as the blood flow in AVF access (Qa) of at least 600 ml/min with draining vein diameter of ≥6 mm at 3 months.
Doppler ultrasonography of arterio-venous fistula
The ultrasonography of AVF was done by an experienced radiologist, working in our hospital. The radiologist was blinded from the clinical history of the patient and also from knowing to which group (Test or control group) patient belonged to. All the measurements were taken using high-frequency probe from a single machine “Esaote My Lab Six.”
The parameters that were measured during an initial visit during recruitment include the following:
-
1)
Radial artery intimal medial thickness (RIMT)
-
2)
Brachial artery intimal medial thickness (BIMT)
-
3)
Radial and brachial artery diameter (in mm)
-
4)
Cephalic vein diameter (in mm).
The parameters that were measured during 4th week and 3rd month follow-up include the following
-
a)
Cephalic vein diameter (mm) – [Figure 1]
-
b)
Radial and brachial artery diameter (mm)
-
c)
AVF diameter (mm)
-
d)
Blood flow through AVF (ml/min)- It was measured at 2.5 cm, 5 cm, and 10 cm from the fistula site, and the average of all the 3 readings was recorded as the flow through AVF
-
e)
Depth of the cephalic vein from the skin (mm).
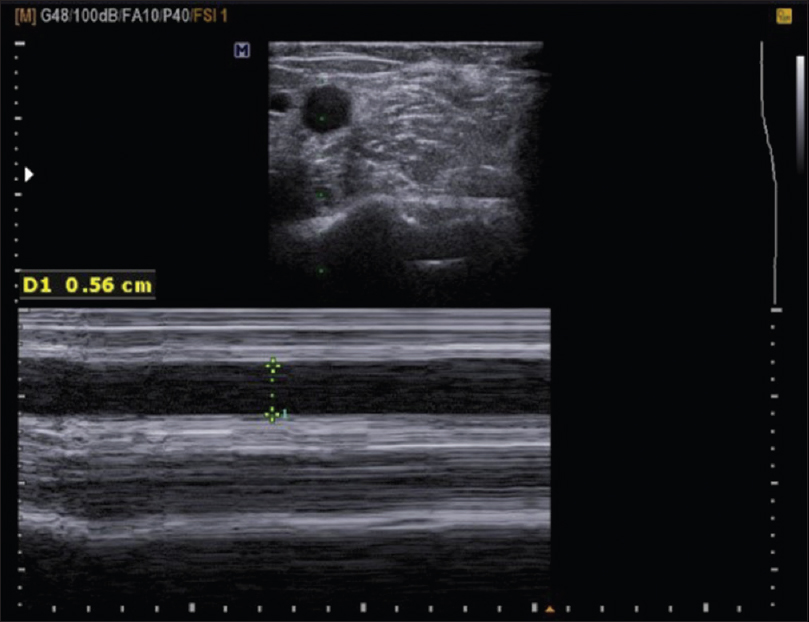
- Post AVF cephalic vein diameter
Apart from these parameters, we also looked for the presence of any atheromatous plaque or inflow/outflow stenosis or thrombosis in arterial/venous limbs of AVF. All these findings were recorded and patients were sent for the interventions, if essential.
Intimal medial thickness: Sum of the thickness of the intimal and the medial layers of the artery taken together [Figure 2]. The normal range for radial artery IMT:<0.25 mm.

- Radial artery intimal medial thickness (Marked as 1)
AVF depth: The distance between the skin surface and the anterior wall of AVF.
Flow in AVF: The exact location for measuring flow in fistula was not known. On the basis of previous studies done by “The Hemodialysis Fistula Maturation” (HFM) group and others, we recorded flow rates in the vein distal to the fistula at 2.5 cm, 5 cm, and 10 cm - took an average of all 3 to note the flow through AVF [Figure 3].
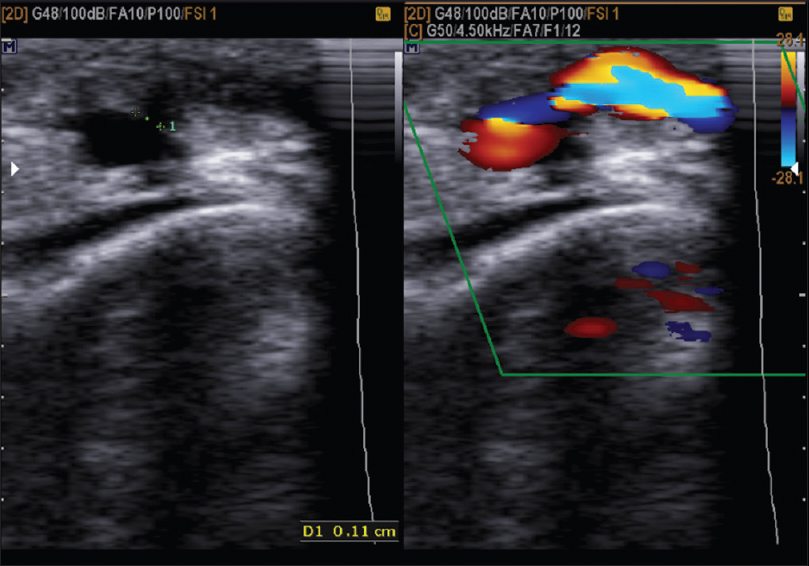
- Flow through AVF and AVF diameter
Far infrared therapy
FIR radiation (λ = 3–100 μm) is a part of the electromagnetic spectrum. This spectrum has been studied in detail for its biological effects. With the advance in technology, specially devised lamps that emit only this spectrum are available and have been shown to promote AVF patency and maturation.
Lamp used: TDP Far Infra Red Lamp (KS 9800).
Distance from patient skin: The lamp was kept at a distance of 30 cm from the patient's skin surface [Figure 4].
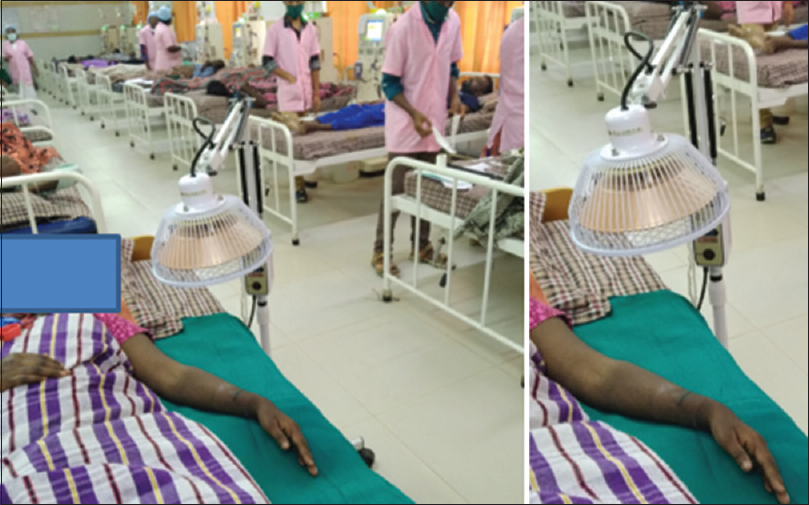
- FIR lamp and its distance (30 cm) from the skin surface
Arterio-venous fistula creation and venous sample
All AVF surgeries were anastomoses between the side of the artery to the end of the vein. All the fistula procedures were done by the vascular surgeons. Approximately, 5–8 mm of the sample from the venous end was collected in formalin during each AVF surgery, and the sample was sent for histo-pathological examination.
Venous biopsy
The venous samples were processed and 2 sections from each sample were taken for analysis. The first section was stained using Hematoxylin and Eosin (H and E) to study the intima thickness, [Figure 5] and the second section was stained using Masson's trichrome to measure the amount of fibrosis in tunica media [Figure 6]. The thickness of intima and media were assessed by capturing images at high-power field (40×) and analyzed with “IMAGES” software. The thickness of intima and fibrosis in media were measured separately in Microns.
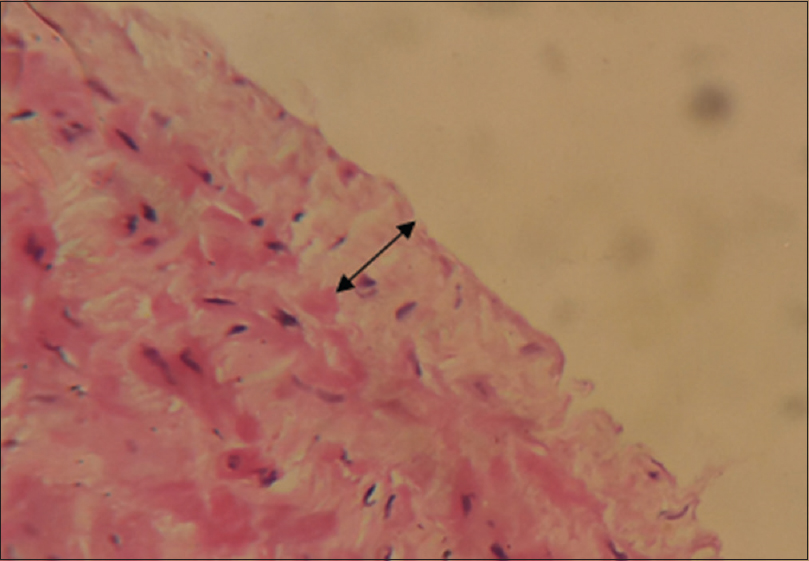
- H and E stain showing thickness of cephalic vein intima (40× Magnification)
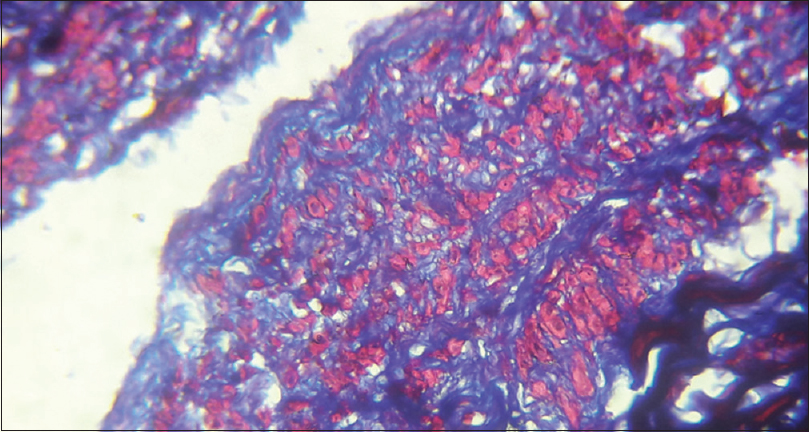
- Trichrome stain showing cephalic vein medial fibrosis (40× Magnification)
Flow of patient in the study is shown in Flow chart 1.
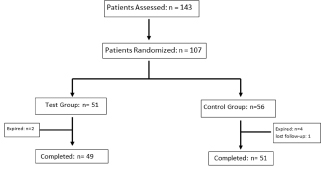
- Flow chart of patients enrolled and completed the study
Statistical analysis
Qualitative variables were summarized as frequencies and proportions. Quantitative variables were summarized as mean and standard deviation after checking for normality of distribution. The difference in flow between the two arms at 4 weeks and 3 months was tested using repeated measures ANOVA. Chi-square test and independent t test were used to test for statistically significant differences between the treatment and control arms. A cut-off of P < 0.05 was used to interpret statistical significance.
Results
Baseline clinical characteristics
Out of 107 patients included in the study, 51 were randomized to the test group and 56 were randomized to the control group. The baseline characteristics of the participants in the test arm and the control arm are compared in Table 1. The number of patients who underwent RCF was 87 and BCF was 20. The number of patients who underwent RCF and BCF in both groups were compared in Table 2.
| Characteristics | Mean±SD/n (%) | P | |
|---|---|---|---|
| Test group | Control group | ||
| Age | 44.5±12.3 | 47.0±14.2 | 0.322 |
| Sex | |||
| Male | 33 (64.7%) | 35 (62.5%) | 0.813 |
| Female | 18 (35.3%) | 21 (37.5%) | |
| Diabetics | 15 (29.4%) | 14 (25.0%) | 0.608 |
| Cardiac Disease | 2 (3.9%) | 1 (1.8%) | 0.504 |
| Smoker | 16 (31.4%) | 13 (23.2%) | 0.343 |
| Alcoholic | 18 (35.3%) | 16 (28.6%) | 0.456 |
| eGFR | 6.32±3.51 | 6.90±4.51 | 0.464 |
| Hb | 8.53±1.66 | 8.31±1.33 | 0.444 |
| Alb | 3.34±0.46 | 3.30±0.47 | 0.674 |
| Ca | 7.93±0.77 | 8.03±0.95 | 0.588 |
| Phosphorus | 5.13±0.93 | 5.33±1.09 | 0.333 |
| Uric acid | 5.39±1.69 | 5.52±1.62 | 0.670 |
| Creatinine | 10.43±4.27 | 9.73±4.84 | 0.431 |
| CCBs | 41 (80.4%) | 50 (89.3%) | 0.422 |
| β- Blocker | 20 (39.2%) | 21 (37.5%) | 0.818 |
| RIMT | 0.39±0.11 | 0.39±0.11 | 0.860 |
| BIMT | 0.42±0.09 | 0.43±0.10 | 0.638 |
| Baseline-Rad A Dia | 2.65±0.65 | 2.48±0.43 | 0.116 |
| Baseline- Brach A Dia | 4.69±0.78 | 4.46±0.75 | 0.128 |
| Baseline- Ceph V Dia | 2.89±0 0.31 | 2.86±0.24 | 0.510 |
| Surgery | CKD Stage | n (%) | ||
|---|---|---|---|---|
| Test Group | Control Group | Total | ||
| RCF | 4 | 4 (9.3%) | 4 (9.1%) | 8 (9.2%) |
| 5 | 39 (90.7%) | 40 (90.9%) | 79 (90.8%) | |
| BCF | 4 | 0 | 1 (8.3%) | 1 (5.0%) |
| 5 | 8 (100.0%) | 11 (91.7%) | 19 (95.0%) | |
Effect of FIR on the AVF flow rate
The blood flow rate in AVF (Qa) at the end of 3 months was divided into 3 categories-
-
i)
Qa <500 ml/min;
-
ii)
Qa- 500 to 800 ml/min and
-
iii)
Qa >800 ml/min.
At the end of the study period (3 months), out of 87 patients who underwent RCF, 70.7% of patients in the test arm and 43.9% of patients in the control arm had Qa >800 ml/min. Similarly, out of the 20 patients who underwent BCF, 6 (75%) patients in test arm and 5 (50%) patients in the control arm had Qa >800 ml/min. The test arm had a higher percentage of patients with high Qa (>800 ml/min) than the control arm (p: 0.003) [Table 3].
| AVF | Flow Rate | Test Group | Control Group | P | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| RCF | <500 | 5 | 12.2% | 12 | 29.3% | |
| 500-800 | 7 | 17.1% | 11 | 26.8% | 0.003 | |
| >800 | 29 | 70.7% | 18 | 43.9% | ||
| BCF | <500 | 1 | 12.5% | 1 | 10.0% | |
| 500-800 | 1 | 12.5% | 4 | 40.0% | 0.142 | |
| >800 | 6 | 75.0% | 5 | 50.0% | ||
The average blood flow rate (Qa) in RCF was 714.05 ± 221.47 and 581.3 ± 179.5 at the end of 4 weeks in the test and the control group, respectively. Qa in RCF at end of 3rd month was 850.38 ± 316.07 and 649.77 ± 367.82 in the test and the control arm [Table 4]. This difference in Qa at the end of the 3 months was statistically significant inferring that the average AVF flow increases with FIR. The average blood flow rate (Qa) in BCF was 832.99 ± 369.42 and 584.62 ± 258.68 at the end of 4 weeks in the test and the control group, respectively. Qa in BCF at end of 3rd month was 980.42 ± 403.29 and 922.12 ± 489.07 in the test and the control arm, respectively. This difference in BCF Qa was statistically not significant. The FIR therapy had no statistically significant influence over the increase in cephalic vein diameter post AVF [Table 5].
| Test Group | Control Group | P | |||
|---|---|---|---|---|---|
| Qa 4th week | Qa 3rd Month | Qa 4th week | Qa 3rd Month | ||
| Surgery | |||||
| RCF | 714.05±221.47 | 850.38±316.07 | 581.30±179.50 | 649.77±367.82 | 0.003 |
| BCF | 832.99±369.42 | 980.14±403.29 | 584.62±258.68 | 922.12±489.07 | 0.410 |
| Cephalic vein diameter | Test group | Control group | P |
|---|---|---|---|
| Baseline | 2.89±0.31 | 2.86±0.24 | 0.510 |
| 4th week | 4.96±0.82 | 4.54±1.11 | 0.62 |
| 3rd month | 6.35±1.26 | 6.16±1.71 | 0.31 |
Effect of far infrared on AVF failure
The number of AVF failures was 5 (10.2%) and 14 (28%) in the test and control arms, respectively (p: 0.025) [Table 6]. In the control arm, 3 patients had a failure of fistula within 6 h of surgery, which may be related to the surgical technique. When adjusted for patients with failure of AVF within 6 h of surgery, the number of AVF failures in test and control arms were 5 and 11, respectively (p: 0.121).
| AVF outcome | OR | P | ||||
|---|---|---|---|---|---|---|
| Success | Failure | |||||
| n | % | n | % | |||
| Test arm | 44 | 89.8% | 5 | 10.2% | 0.292 (0.096,0.888) | 0.025 |
| Control arm | 36 | 72.0% | 14 | 28.0% | ||
Baseline arterial intima-media thickness and AVF outcome at the end of 3 months
The mean IMT of the radial artery for patients who underwent RCF was 0.39 ± 0.12 mm in the test arm and was 0.39 ± 0.11 mm in the control arm. Similarly, the mean IMT of the brachial artery for the patients who underwent BCF was 0.49 ± 0.10 mm in the test arm and was 0.47 ± 0.11 mm in the control arm. As shown in Table 7, the mean Qa was high in patients with the arterial IMT <0.5 mm when compared with those with IMT ≥0.5 mm. This difference in flow was significant statistically in patients with RCF (p–0.003) and also in patients with BCF (p–0.014) inferring that the average flow through AVF was significantly high if the IMT <0.5 mm. The arterial IMT of the anastomosed artery had statistically significant correlation with the primary failure rate of AVF (P < 0.001).
| Test Group | Control Group | P | |||
|---|---|---|---|---|---|
| 4th week- Qa (Mean±SD) | 3rd month- Qa (Mean±SD) | 4th week- Qa (Mean±SD) | 3rd month- Qa (Mean±SD) | ||
| RIMT | |||||
| <0.5 mm | 801.98±172.65 | 940.45±264.60 | 614.10±181.18 | 815.64±348.62 | 0.003 |
| >=0.5 mm | 466.27±330.36 | 602.93±432.05 | 483.16±212.50 | 374.41±384.90 | |
| BIMT | |||||
| <0.5 mm | 751.49±188.08 | 887.43±309.16 | 594.03±203.34 | 734.56±392.58 | 0.014 |
| >=0.5 mm | 688.41±368.92 | 831.90±388.96 | 560.42±184.40 | 645.62±429.55 | |
Baseline Cephalic vein Intima- Media thickness and AVF outcome
Out of 107 patients who were randomized, we were able to collect sample from the venous end in 88 patients. Out of these 88 samples, 24 were inadequate for further processing and hence were discarded. Remaining 64 samples were processed and cut into thin sections and were stained with H and E and Masson's trichrome stains. Final interpretation using the IMAGES software was possible only in 48 samples, which were stained with H and E and only for 41 samples, which were stained using trichrome. The number of samples that were interpreted with both the stains was 33, with 21 in the control arm and 12 in the test arm. As this number was too low when compared to the sample size, we were unable to correlate the AVF outcome with the baseline cephalic vein diameter. The maximum cephalic vein intima thickness measured was 54.9 μm and the maximum fibrosis in tunica media of the venous wall was 24.4 μm.
Risk factors associated with AVF failure
The only risk factor, other than IMT, which was found to be associated with poor AVF outcome, was the presence of diabetes mellitus. Its association with AVF primary failure was statistically significant (P: 0.004). Statistically, all the other known risk factors such as smoking, female sex, obesity, and advanced stage of CKD were not found to be associated with AVF primary failure [Table 8].
| AVF outcome | OR | P | ||||
|---|---|---|---|---|---|---|
| Success | Failure | |||||
| n | % | n | % | |||
| Sex | ||||||
| Male | 51 | 79.7% | 13 | 20.3% | 0.812 (0.279,2.365) | 0.702 |
| Female | 29 | 82.9% | 6 | 17.1% | ||
| Diabetes | ||||||
| No | 64 | 87.7% | 9 | 12.3% | 4.44 (1.55,12.75) | 0.004 |
| Yes | 16 | 61.5% | 10 | 38.5% | ||
| Smoker | ||||||
| No | 60 | 83.3% | 12 | 16.7% | 1.75 (0.61,5.1) | 0.297 |
| Yes | 20 | 74.1% | 7 | 25.9% | ||
| Alcoholic | ||||||
| No | 54 | 80.6% | 13 | 19.4% | 0.959 (0.327,2.807) | 0.638 |
| Yes | 26 | 81.2% | 6 | 18.8% | ||
| Stage | ||||||
| 4 | 7 | 77.8% | 2 | 22.2% | 0.815 (0.155,4.28) | 0.809 |
| 5 | 73 | 81.1% | 17 | 18.9% | ||
| Surgery | ||||||
| RCF | 64 | 79.0% | 17 | 21.0% | 0.471 (0.098,2.249) | 2.336 |
| BCF | 16 | 88.9% | 2 | 11.1% | ||
Discussion
Vascular access is rightly known as the Achilles' heel of hemodialysis.[17] For a successful AVF, preservation of veins and choosing vessels that are suitable for AVF are very essential. The healthy vein, when anastomosed with an artery, will usually undergo outward remodeling, but adequate maturation may be interfered by pre-existing vasculopathy, like intimal hyperplasia in patients with CKD. In spite of this intimal hyperplasia, adequate outward remodeling may result in intact-caliber of the lumen, but if intimal hyperplasia outbalances the outward remodeling, it may result in stenosis, which finally ends in failure of AVF. IMT is used as a surrogate marker of intimal hyperplasia, and increased arterial IMT has been shown to be associated with poor AVF outcome.[18] In 2006, Ku et al. showed that the measurement of IMT in the artery in patients with CKD by ultrasonography correlates well with the histological documentation of intimal hyperplasia.[19]
Although multiple agents/therapies were tried to accelerate and to facilitate the AVF remodeling, none was found effective. Hence, there is a constant search for newer strategies to improve the patency of fistula. Although the role of FIR therapy in accelerating the cumulative unassisted patency rate was known for the past 10 years, there was no large randomized control trial available till date. Most of the studies available till date to convey the role of FIR therapy in AVF maturation were conducted in Taiwan and China.[20212223] FIR is a part of infrared (IR) band of the electromagnetic spectrum [Figure 7].
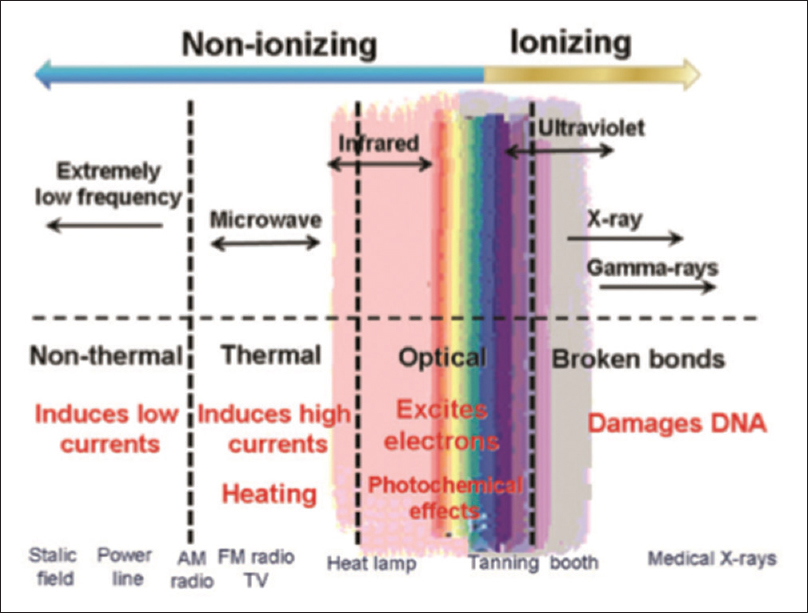
- The spectrum of electromagnetic radiation and some biological changes it may induce[24]
On the basis of the wavelength, ISO 20473 standards subdivided IR ranges into 3 divisions: near IR (0.78–3 μm), mid IR (3–50 μm), and FIR (50–1000 μm). Among these, only FIR rays are known to transfer energy as heat.[25] The wavelength of FIR is too long to be seen by naked eyes but may be felt as heat, that can penetrate up to 4 cm beneath the skin.
FIR therapy has been proved to ameliorate endothelial dysfunction and thereby improve vascular health. It was shown that the coronary perfusion was improved with FIR therapy, which may be related to the up-regulation of endothelial nitric oxide synthase in the endothelium resulting in the betterment of cardiac function.[26] The FIR therapy also has been proved to be useful in reducing oxidative stress in diabetics, promoting wound healing, and reducing easy fatigability in patients with CKD.[27282930] Lamps and saunas, delivering pure FIR radiation (eliminating completely the near and mid-IR bands), have become safe, effective, and widely used sources to generate therapeutic effects.
In this study, the AVF maturation was measured by assessing 2 parameters namely, the blood flow rate through AVF (Qa) and the diameter of the cephalic vein draining (CVd) the AVF. At the end of 3 months, Qa in RCF was 850.38 ± 316.07 and 649.77 ± 367.82 in the test and the control group, respectively. This difference in Qa at the end of the 3 months is statistically significant inferring that the average AVF flow increases with FIR (p- 0.003). This is comparable to the previous study by Lee et al.[22] published in 2013.
At the end of 3 months, out of 87 patients who underwent RCF earlier, 70.7% of patients in the test arm and 43.9% of patients in the control arm had blood flow rate in AVF (Qa) >800 ml/min. Similarly, out of the 20 patients who underwent BCF, 6 (75%) patients in the test arm and 5 (50%) patients in the control arm had Qa >800 ml/min. This difference in the percentage of participants with high-flow rate is statistically significant. (p–0.003). The results are comparable to the study by Lee et al.[22] The mean CVd at the end of 3 months was 6.35 ± 1.26 mm and 6.16 ± 1.71 mm in the test and the control arm, respectively at the end of 3 months. This difference was statistically not significant.
At the end of 3 months, the numbers of AVF failures were 5 (10.2%) and 14 (28%) in the test and control groups, respectively, which was statistically significant (p–0.025). However, when adjusted for patients with failure of AVF within 6 h of surgery (which may be related to the surgical technique) this difference in the AVF patency was statistically insignificant (p–0.121). The mean Qa achieved in AVF at the end of 3 months was high in patients with arterial IMT <0.5 mm when compared with those with IMT ≥0.5 mm. This difference in flow is significant statistically in patients with RCF (p–0.003) and also in patients with BCF (p–0.014) inferring that the average flow through AVF is significantly high if the IMT <0.5 mm.
In the current study, the IMT of the anastomosed artery has statistically significant correlation with the primary failure rate of AVF (P < 0.001). This result is similar to the study by Kim et al. in 2006, which showed that in patients with IMT ≥0.5 mm, AVF failure within 1 year was higher than in patients with IMT <0.5 mm (72% vs. 50%, P = 0.017). This difference in our study compared with the other RCTs is tabulated in Table 9.
| Study | Decide | Patients | FIR exposure | Study Duration (Months) | Results |
|---|---|---|---|---|---|
| Choi et al.[31] | RCT | FIR: 25 Control: 25 | 40 min Thrice weekly | 12 | FIR therapy increased AVF blood flow from 881.6 to 934.7 ml/min |
| Lai et al.[32] | RCT | FIR: 118 Control: 98 | 40 min Thrice weekly | 12 | No statistical difference in unassisted patency rate at 1 year between groups (25.0% versus 18.4%) |
| Lin et al.[20] | RCT | FIR: 72 Control: 73 | 40 min Thrice weekly | 12 | FIR reduced AVF malfunction (12.5% versus 30.1%), and increased unassisted patency rate of AVF (85.9% versus 67.6%) |
| Lin et al.[21] | RCT | FIR: 139 Control: 141 | 40 min Thrice weekly | 12 | FIR can improve the unassisted patency rate compared with the control group (87.4% versus 72.5%) |
| Lin et al.[22] | RCT | FIR: 20 Control: 20 | 40 min Thrice weekly | 12 | FIR improves the access flow, improve the unassisted patency rate (87% versus 70%), decrease AVF malfunction (12% versus 29%) |
| Yang et al.[23] | RCT | FIR: 20 Control: 20 | 40 min Thrice weekly | 6 | FIR improves the access blood flow and decrease inflammation |
| Current study | RCT | FIR: 51 Control: 56 | 40 min Twice weekly for 4 weeks | 3 | FIR therapy improves access flow (850.38±316.07 ml/min vs. 649.77±367.82ml/min) and decreases AVF primary failure (10.2% vs. 28%) |
Limitation: Our study had small sample size. We need a larger sample size and longer and more frequent exposure to the FIR to find its role in promoting AVF maturation.
Conclusion
We conclude that in the South Indian population with CKD (stage 4 and 5), FIR therapy is effective in increasing the AVF blood flow rate at the end of 3 months, though the difference in the primary failure rate is statistically insignificant when adjusted for patients with AVF failure within 6 h of surgery, which may be related to the surgical technique. We also found that the IMT of the anastomosed artery has the impact on the primary failure rate of AVF. The only other risk factor associated with poor AVF outcome is the presence of diabetes mellitus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
I would like to acknowledge, Mr. T. Parthiban- Senior Dialysis technician and Mrs. Kanagalakshmi- Dialysis technician, for their support in assisting us in administering FIR therapy to all the patients in the test group.
References
- NKF-K/DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S248-73.
- [Google Scholar]
- Dialysis Access Consortium Study Group: Effect of clopidogrel on early failure of arterio-venous flstulas for hemodialysis: A randomized controlled trial. JAMA. 2008;299:2164-71.
- [Google Scholar]
- Dialysis flstula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol. 2010;5:2348-54.
- [Google Scholar]
- Hemo-dialysisarteriovenousflstulapatency revisited: Results of a prospective, multi-centerinitiative. Clin J Am Soc Nephrol. 2008;3:714-9.
- [Google Scholar]
- Outcomes of arteriovenous flstula creation after the Fistula First Initiative. Clin J Am Soc Nephrol. 2011;6:1996-2002.
- [Google Scholar]
- When is a new fistula mature? The emerging science of fistula cannulation. Semin Nephrol. 2012;32:564.
- [Google Scholar]
- Type of arteriovenous vascular access and association with patency and mortality. BMC Nephrol. 2013;14:79.
- [Google Scholar]
- Vascular access and all-cause mortality: A propensity score analysis. J Am Soc Nephrol. 2004;15:477-86.
- [Google Scholar]
- A comparison of arteriovenous fistulas and central venous lines for long-term chronic haemodialysis. Pediatr Nephrol. 2013;28:321-6.
- [Google Scholar]
- Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA. 2008;299:2164-71.
- [Google Scholar]
- Collaborative overview of randomised trials of antiplatelet therapy-II: Maintenance of vascular graft or arterial patency by antiplatelet therapy: Antiplatelet Trialists collaboration. BMJ. 1994;308:159-68.
- [Google Scholar]
- Effects of far-infrared ray on reproduction, growth, behaviour and some physiological parameters in mice. In Vivo. 2000;14:321-6.
- [Google Scholar]
- Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photo Med. 2006;22:78-86.
- [Google Scholar]
- Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J. 2006;70:463-70.
- [Google Scholar]
- World Medical Association Declaration of Helsinki. Available from: http://wwwwmanet/e/policy/b3htm
- Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol. 2008;28:739-45.
- [Google Scholar]
- Arteriovenous access failure: More than just intimal hyperplasia? Nephrol Dial Transplant. 2013;28:1085-92.
- [Google Scholar]
- Ultrasonographic measurement of intima-media thickness of radial artery in pre-dialysis uraemic patients: Comparison with histological examination. Nephrol Dial Transplant. 2006;21:715-20.
- [Google Scholar]
- Far-infrared therapy: A novel treatment to improve access blood flow and unassisted patency of arteriovenous fistula in hemodialysis patients. J Am Soc Nephrol. 2007;18:985-92.
- [Google Scholar]
- Length polymorphisms of heme oxygenase-1 determine the effect of far-infrared therapy on the function of arteriovenous fistula in hemodialysis patients: A novel physicogenomic study. Nephrol Dial Transplant. 2013;28:1284-93.
- [Google Scholar]
- Effect of far infrared therapy on arteriovenous fistula maturation: An open label randomized controlled trial. Am J Kidney Dis. 2013;62:304-11.
- [Google Scholar]
- Clinical observation of far infrared radiation on autogenous arteriovenous fistula in maintenance hemodialysis patients. Chin J Pract Internal Med. 2015;35:1019-22.
- [Google Scholar]
- FIR: Its biological effects and medical applications. Photon Lasers Med. 2012;1:255-66.
- [Google Scholar]
- The fine tuning of pain thresholds: A sophisticated double alarm system. PLoS One. 2010;5:e10269.
- [Google Scholar]
- Repeated thermal therapy upregulates arterial endothelial nitric oxide synthase expression in Syrian golden hamsters. JpnCirc J. 2001;65:434-8.
- [Google Scholar]
- The effect of leg hyperthermia using far infrared rays in bedridden subjects with type 2 diabetes mellitus. Acta Med Okayama. 2010;64:143-7.
- [Google Scholar]
- Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp Biol Med (Maywood). 2003;228:724-9.
- [Google Scholar]
- Effects of far infrared acupoint stimulation on autonomic activity and quality of life in hemodialysis patients. Am J Chin Med. 2009;37:215-26.
- [Google Scholar]
- Clinical utility of far infrared therapy for improvement of vascular access blood flow and pain control in hemodialysis patients. Kidney Res Clin Pract. 2016;35:35-41.
- [Google Scholar]
- Post-angioplasty far infrared radiation therapy improves 1-year angioplasty-free hemodialysis access patency of recurrent obstructive lesions. Eur J Vasc Endovasc Surg. 2013;46:726-32.
- [Google Scholar]







