Translate this page into:
Naproxen Induced Acute Interstitial Nephritis with Renal Cortical Necrosis
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Drug induced acute interstitial nephritis is an idiosyncratic reaction following a drug exposure. The commonest drugs implicated are nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics and proton pump inhibitors. Renal cortical necrosis is a rare cause of acute kidney injury caused by severe and sustained vasoconstriction of small renal vessels. There is a change in the epidemiology of acute kidney injury especially in developing countries where drug induced acute kidney injury is becoming increasingly common. Naproxen is known to cause renal failure by renal papillary necrosis, tubular damage and acute interstitial nephritis. We present a case of Naproxen induced acute interstitial nephritis with acute cortical necrosis. To the best of our knowledge this is the first documented case of Naproxen induced renal cortical necrosis.
Keywords
Renal cortical necrosis
Naproxen
NSAIDs
Introduction
Significant changes are observed in the epidemiology of acute kidney injury (AKI) in the past decade. Sepsis and shock is the commonest cause of AKI, especially in developing countries.[1] With better health care and quality of life, now there is a change in epidemiology; more cases of drug induced renal injury are being reported.[2] Incidence of renal cortical necrosis (RCN) is 1.9-2% of all AKI, the commonest cause being obstetric complications.[3] Drugs especially non-steroidal anti-inflammatory drugs (NSAID's) are very rarely described in the literature to cause cortical necrosis.[45]
Case Report
A 62-year-old gentleman, presented with fever, nausea, vomiting and bilateral flank pain for 2 days. He had bilateral pedal oedema and was breathless. He is known to have Type 2 diabetes mellitus for 30 years, with poorly controlled blood sugars (HbA1c - 10%), bilateral non-proliferative diabetic retinopathy and sub-nephrotic range proteinuria with normal serum creatinine. He had systemic hypertension for ten years and was on Losartan (50 mg once daily) for control of blood pressure. He had taken Naproxen 500 mg twice daily (for migraine) for one week prior to admission. He had no myalgia, icterus, skin or mucosal bleeds, rash, or arthritis. He had no dysuria, haematuria, or lower abdominal pain. No history of haemoptysis or blood in stools, chest pain or hypotensive episodes. He did not have productive cough, sore throat, skin lesions or other focus of infections; no abdominal pain radiating to back.
On examination, he was febrile, tachypneic with a blood pressure of 170/90 mmHg. He had bilateral pedal oedema and bilateral basal coarse crepitations on auscultation of chest.
Investigations revealed serum creatinine of 3.1 mg/dl, serum Sodium: 127 meq/L, serum potassium: 5.4 meq/L, metabolic acidosis (pH 7.28, HCO3 18.9, pCO2 35.7, Lactate 1.93); random blood sugar was 200 mg/dl. His haemoglobin was 10.1 g/dL, total count 19,800/Cu. mm with neutrophils 75%, lymphocytes 2.9%, eosinophils 14.5% and platelet count 1.8 L/cu mm. He had eosinophilic leucocytosis with absolute eosinophil count of 2800/Cu. mm (normal 20-500/Cu mm) and eosinophiluria (urine eosinophils 39%). Liver function test and Creatinine phosphokinase levels (65.6 mg/dl) were normal. Ultrasonogram of abdomen showed mildly enlarged kidneys (right kidney 12.5 cm and left kidney 12 cm); both kidneys were hyperechoeic in echotexture with normal pelvicalyceal system. X ray chest was normal and echocardiogram did not show any vegetations. Plain CT abdomen showed mildly enlarged kidneys and normal collecting system.
With the above clinical picture, a provisional diagnosis of acute pyelonephritis was considered and broad-spectrum parenteral antibiotics started. However, there were no urinary tract symptoms, ultrasonogram of abdomen ruled out obstructive uropathy or any collection or abscess. Meanwhile, urine microscopy showed 3 + albumin, RBC 1/hpf, and WBC 24/hpf. There were no cast or crystals; blood and urine cultures were sterile. C reactive protein was only mildly elevated (55.75 mg/dl). Stool routine and microscopy did not show any ova or cysts. Peripheral blood smear was negative for haemoparasites, atypical cells or schistocytes. There were no features of hemolysis on blood smear. Other infective causes like Leptospirosis, Dengue fever and Malarial illness were ruled out with appropriate tests. There were no features of Raynaud's phenomenon, livedo reticularis, rash or Hollerhorst plaque on ophthalmic fundus examination. History was reviewed and there was no history of ingestion of any other medications (other than Naproxen and Losartan), native medicines, and poisonous substances. There was no history of animal, insect or snake bite, and no recent severe physical exertion either. There was no evidence of acute pancreatitis on blood tests or on abdominal imaging.
Renal failure worsened over a period of two days and he became oligoanuric with severe uremic symptoms and worsening breathlessness. He was initiated on haemodialysis via non-tunneled internal jugular venous catheter. The patient had persistent fever spikes in spite of broad-spectrum antibiotic and his urine culture and blood cultures were sterile, with no other evidence of sepsis. Serial blood counts showed a progressive increase in eosinophils from 18.7 to 35.7% (AEC 2880 to 6450 cells/cubic mm). In view of eosinophilia, drug induced fever was considered, and antibiotics were stopped.
Renal functions did not improve over a period of ten days and a renal biopsy was done, which showed patchy cortical necrosis [Figure 1a] and dense eosinophilic infiltrate admixed with lymphoplasmacytic inflammatory infiltrates and focal interstitial edema [Figure 1b], suggestive of acute interstitial nephritis. There was no interstitial granuloma seen. There was no endocapillary proliferation or crescents. There was no evidence of capillary thrombi suggestive of hemolytic uremic syndrome or any features of vasculitis. However, afferent and efferent arterioles showed medial hypertrophy and hyaline arteriosclerosis. Immunofluorescence test was negative for IgG, IgA, IgM, C3, C1q, kappa, lambda light chains and fibrinogen. There were no intra-parenchymal crystals seen. Doppler of renal vessels was normal and did not show any thrombus that could embolise to kidneys.
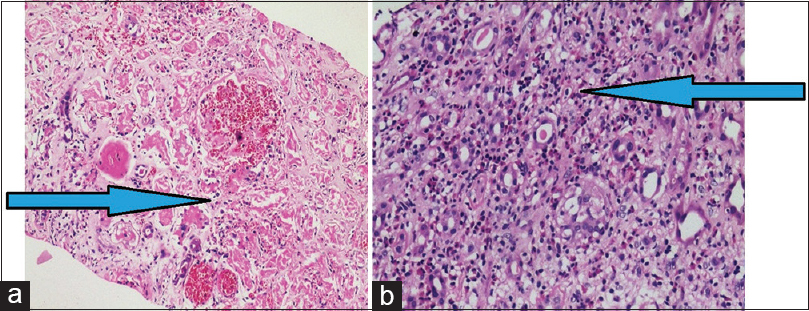
- Renal biopsy with patchy cortical necrosis (arrow) (a, H and E, 40X); with dense interstitial infiltrates of eosinophils and lymphocytes (arrow) (b, H and E,40X)
The patient was started on oral Prednisolone (1 mg/Kg) and he was continued on dialysis. His urine output steadily increased, serum creatinine improved to 2.6 mg/dL and he was off dialysis after 6 weeks. Steroid was tapered and stopped.
Discussion
The incidence of AKI has been increasing recently and significant changes are observed in the past decade.[1] There is a change in epidemiology of AKI - the shift of etiology from infectious causes, sepsis and obstetric causes to non-infectious causes like drugs. In a study conducted in Eastern India (1996-2008), in an analysis of 2405 cases, there has been significant increase in drug induced renal failure, mostly caused by NSAIDs and Rifampicin.[2] NSAIDs are one of the most abused drugs in the community and in a hospital setting that can cause significant renal injury.
Acute interstitial nephritis (AIN) accounts for 15-27% of patients with acute kidney injury,[6] whereas renal cortical necrosis is much rarer entity accounting for only 1.9%-2% of all patients with AKI.[4] Acute interstitial nephritis is caused by drugs, infections and toxins. It is more common with antibiotics, NSAIDs and proton pump inhibitors. It was classically described with Methicillin as a triad of fever, eosinophilia and skin rash which is present in less than 10% of cases. NSAID induced acute interstitial nephritis can have more prominent renal failure and proteinuria in the absence of skin rash or other systemic symptoms[6] and can have arthralgia and microscopic haematuria. Our patient had most features of AIN - fever, eosinophilic leucocytosis, eosinophiluria, and AKI with fever responding to stopping of offending drug.
The presence of concomitant patchy renal cortical necrosis in a case of acute interstitial nephritis due to NSAIDs is an unusual and unexpected finding. Renal cortical necrosis is caused by intense, severe and sustained vasoconstriction of small renal vessels like afferent arteriole, renal artery thrombosis causing embolic showers or endothelial damage or coagulopathy causing thrombosis that results in severe ischaemic necrosis of patches of glomeruli and tubules.[3] It is a rare cause of AKI (1.9%-2%). Obstetric complications were the main causes (60-70%) of RCN in developing countries. The remaining cases of RCN caused by non-obstetric causes were mostly due to sepsis and haemolytic uremic syndrome. It generally has a poor prognosis. However, with improvement in healthcare, the epidemiology is slowly changing. The non-obstetric conditions leading to acute cortical necrosis include snake bite (sea snake, green pit viper, Russell viper) (14.2% of all cases of acute renal cortical necrosis in the study),[4] Haemolytic Uremic Syndrome (11.5%), hyper acute kidney rejection in transplant recipients, acute gastroenteritis (4.4%), acute pancreatitis (3.5%), septicemia (2.7%) and drugs (0.9%). Other causes of ACN include shock, extensive burns, diabetic keto-acidosis, multiple fractures, haemorrhage, other infections like Leptospirosis, Plasmodium falciparum, Meningococcal meningitis, Acquired anti protein S deficiency, post Varicella infection, SLE related and primary anti-phospholipid antibody syndrome, wasp sting, dehydration in infancy or childhood, intra-abdominal procedures, sickle cell crisis and cryoglobulinemia.
Drug-induced cortical necrosis is very rare (0.9% of acute cortical necrosis). Many toxins and drugs are implicated in causing cortical necrosis like hypophosphatemia and myoglobinuria in rhabdomyolysis (severe physical exertion),[7] Tranexamic acid,[8] ethanol[9] and contrast dye.[10] NSAID induced cortical necrosis is even rarer.[511] In our case no other cause of acute cortical necrosis could be found except NSAIDs. NSAIDs cause both immune mediated damage and non-immune mediated damage to the kidneys. Immune mediated damage is due to immunological reaction against endogenous nephritogenic antigens or exogenous antigens processed by tubular cells with cell mediated immunity having a pathogenic role. Mechanism of non-immune mediated damage is by various mechanisms mostly by non-selective Cyclooxygenase inhibition. NSAID is hypothesised to have caused this intense vasoconstriction by release of cytokines like Endothelin 1 and blocking prostaglandins.
Naproxen is a non-selective Cyclooxygenase inhibitor, known to cause renal failure by renal papillary necrosis, tubular damage and interstitial nephritis.[12] To the best of our knowledge, this is the first reported case of Naproxen-induced renal cortical necrosis.
Conclusion
The presence of renal cortical necrosis and acute interstitial nephritis due to NSAIDs in the same patient is very rare. A high index of suspicion is essential to detect this condition early as it has prognostic implications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Drug-induced kidney disease in the ICU: Mechanisms, susceptibility, diagnosis and management strategies. Curr Opin Crit Care. 2017;23:484-90.
- [Google Scholar]
- Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: A single-centre experience of 22 years from Eastern India. Nephrol Dial Transplantat. 2007;22:1213-7.
- [Google Scholar]
- Nonsteroidal anti-inflammatory drugs and acute cortical necrosis. Ann Intern Med. 1986;105:303-4.
- [Google Scholar]
- Glomerular, tubular and interstitial nephritis associated with non-steroidal antiinflammatory drugs. Evidence of a common mechanism. Br J Clin Pharmacol. 1999;47:203-10.
- [Google Scholar]
- Acute kidney injury due to acute cortical necrosis following a single wasp sting. Ren Fail. 2013;35:170-2.
- [Google Scholar]
- Tranexamic-acid-induced acute renal cortical necrosis in a patient with haemophilia. A Nephrol Dial Transplant. 2001;16:189-90.
- [Google Scholar]
- Bilateral renal cortical necrosis following binge drinking. Alcohol Alcohol. 2012;47:140-2.
- [Google Scholar]
- Vascular causes of renal failure. Renal Failure Developments in Nephrology. 1995;37:55-6.
- [Google Scholar]
- Bilateral renal cortical necrosis following pyrazolone treatment. Dtsch Med Wochenschr. 1967;92:1075-7.
- [Google Scholar]
- Naproxen sodium (Anaprox): Pharmacology, pharmacokinetics and drug interactions. J Reprod Med. 1980;25(4 Suppl):222-5.
- [Google Scholar]







