Translate this page into:
Treatment of Post-biopsy Arteriovenous Fistula of a Renal Graft by Selective Embolization
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The development of an arteriovenous fistula (AVF) after renal graft biopsy is a rare complication, it is associated in most cases with spontaneous resolution. However, interventional therapies are required in some cases, to prevent graft loss. Selective embolization has been described as an alternative treatment. In the present study, we describes our experience on AVF after biopsy in kidney transplant patients, which was managed with selective embolization. From 2005 to 2015, a total of 452 kidney transplant biopsies were performed, 12 had an AVF requiring embolization. In 92% of cases, this was successful. Beforehand, mean serum creatinine levels were 2.45 mg/dL, after the procedure, that increased to 3.05, however, 3 months later, mean creatinine levels dropped to 1.85 mg/dL. Graft survival after 2 follow-up years was 72%. Our experience demonstrates that selective embolization of the AVF after kidney transplant biopsy is a safe procedure, and that transplant function can be maintained in patients with this complication.
Keywords
Arteriovenous fistula
kidney transplantation
graft biopsy
graft rejection
selective embolization
Introduction
Renal biopsy is recognized as the gold standard for the evaluation of renal graft dysfunction; this allows for differential diagnosis and treatment of multiple conditions that can be associated.[1] Despite its invasive nature, percutaneous renal biopsy is a relatively safe procedure,[2] with a low incidence of vascular complications, ranging from 0.2–2% of patients, including bleeding and the development of arteriovenous fistulas (AVFs).[23] The incidence of AVF after kidney graft biopsy is reported in up to 16% of cases, a complication that largely improves spontaneously without any therapy.[2] However, sometimes it can lead to graft dysfunction secondary to the compromise of renal parenchymal perfusion, which requires prompt treatment to avoid graft loss.[4]
Risk factors associated with the development of AVF are uncontrolled arterial hypertension, nephroangiosclerosis, allograft fibrosis, large needle size, penetration into the medulla, coagulation abnormalities, and certain immunosuppressant drugs[56]; Regarding treatment, due to frequent spontaneous resolution, some groups suggest conservative management; however, there are some signs and symptoms that support early intervention, including increased size of the AVF in the follow-up with Doppler, impaired graft perfusion, difficult to control post-biopsy hypertension, and persistent hematuria.[2] Endovascular therapy is among the therapeutic options for this complication[3]; in the case of uncontrolled bleeding, a nephrectomy may be necessary.[4]
There are few reports of embolization in patients with a post-biopsy AVF of a renal graft.[2] Our study aimed to describe our short- and long-term clinical experience with patients who underwent AVF after kidney graft biopsy.
Methods
Retrospective cohort where renal transplanted patients with a diagnosis of AVF secondary to renal graft biopsy were evaluated and managed with selective embolization during the years from 2005 to 2015 at the Pablo Tobón Uribe Hospital (HPTU, Spanish initials), were taken.
Included patients were older than 18 years. The following variables were examined: baseline and demographic characteristics; etiology of the kidney disease; type of donor (living or deceased); immunosuppressive therapy used; an indication of renal biopsy; renal function before biopsy; findings on Doppler of the graft, both pre- and post-biopsy; the number of punctures in the biopsy; the number of samples compatible with renal tissue; hemoglobin and hematocrit pre- and post-biopsy; systolic and diastolic blood pressure at the beginning of the biopsy; signs and symptoms suggestive of AVF; histological diagnosis of the biopsy; treatments received; complications; the time between biopsy and embolization; graft loss; renal function at 3, 6, 12, and 24 months after biopsy; reentry to dialysis; and death.
Data were obtained from the patients' electronic medical records; they were recorded in an Excel database, and then exported to SPSS (Chicago, IL, USA) for statistical analysis.
Standard precaution for graft biopsy were taken. Ultrasound-guided biopsy at one of the poles of the renal graft, preferably from the lateral or upper pole using biopsy gun (ProMag™ Biopsy Needle, 18-gauge X 25 cm, Ref. 765018250 Argon Medical Devices, Frisco, TX, USA). Absolute rest for 6 h with monitoring of vital signs, puncture site, and urine characteristics. Routine subsequent Doppler ultrasound control was not performed. The diagnosis of post-biopsy AVF was made on Doppler ultrasound. Embolization was indicated in the case of impaired graft function or a significant increase in the AVF.
Results
From 2005 to 2015, 452 allograft biopsies were performed, in which 12 patients developed AVF, requiring embolization. Six were men and six women, with a median age of 42 years (p25–75: 36–50.5). Four (33.3%) patients presented with macroscopic hematuria and seven (58.3%) with renal dysfunction. Eleven patients (91.7%) received induction therapy with all received triple renal immunosuppressive therapy [Table 1]. The indication for renal biopsy was graft dysfunction in all patients. Acute graft rejection was confirmed in 83.3% of patients [Table 1]. Kidney biopsy was performed, on average, 2.92 months (percentiles 25–75: 0.34–48.5) after renal transplantation; mean follow-up time from the biopsy until the last consultation was 42 months (p25–75: 5.25–52.25). Other characteristics that were evaluated can be found in Table 1.
| Variables | n (%) or median (p25 - 75) |
|---|---|
| Males % (n) | 50% (6) |
| Females % (n) | 50% (6) |
| Age in years; median (p25-75) | 42 (36-50.5) |
| Second renal transplant % (n) | 16.7% (2) |
| Dyslipidemia background n (%) | 50% (6) |
| Immunosuppression protocol before renal biopsy n (%) | |
| Tacrolimus, MMF, Prednisolone | 75% (9) |
| Cyclosporine, AZA, Prednisolone | 8.3% (1) |
| Everolimus, MMF, Prednisolone | 8.3% (1) |
| Everolimus, Cyclosporine, Prednisolone | 8.3% (1) |
| Delayed renal graft function n (%) | 25% (3) |
| Indication for renal biopsy n (%) | |
| Increase in serum creatinine with suspected rejection | 100% (12) |
| Antithrombotic therapy before biopsy n (%) | 0 (%) |
| number of attempted punctures median (p25-75) (min-max) | 2 (2-3) (2-5) |
| Number of samples of kidney tissue taken median (p25-75) (min-max) | 2 (2-2) (1-3) |
| Rejection confirmed by biopsy | 91.7% (11) |
| The time between transplantation and renal biopsy in months; median (p25-75) | 2.92 (0.34-48.5) |
| The time between biopsy to the detection of arteriovenous fistula; median (p25-75) | 8.5 (3.25-46) |
| The time between biopsy to the last follow-up in months median (p25-75) | 42 (5.25-52.25 |
Patients had an average of two punctures for each renal biopsy. The rejection was reported in 11 (91.7%), of which 4 were cellular rejection, 6 humoral, and 1 mixed rejection. The median time interval between the renal biopsy and diagnosis of AVF was 8.5 months (p25–75: 3.25–46). In 75% of them, renal doppler showed compromised renal flow due to fistula, 25% showed a significant increase in fistula size. The velocity of renal flow in cm/s had a median value of 276.5 (p25–75: 169.5–360). Some laboratory parameters before and after the renal biopsy are shown in Table 2.
| Variables | Median (p25-75) |
|---|---|
| Creatinine (mg/dL) before renal biopsy; median (p25-75) | 2.88 (1.80-7.08) |
| Creatinine (mg/dL) at the time of detection of AV fistula median (p25-75) | 2.45 (1.55-7.19) |
| Creatinine (mg/dL) 48 h post embolization; median (p25-75) | 3.05 (1.50-7.18) |
| GFR at the time of detection of AV fistula median (p25-75) | 1.85 (7.75-47.5) |
| GFR before renal biopsy; median (p25-75) | 19.4 (8.58-38.68) |
| GFR at the time of detection of AV fistula median (p25-75) | 27.0 (7.75-47.5) |
| GFR 48 h after embolization; median (p25-75) | 23.8 (6.9-49.5) |
| Hb (mg/dL) pre renal biopsy; median (p25-75) | 9.85 (8.52-11.35) |
| Ht (%) pre renal biopsy; median (p25-75) | 29.5 (24.65-34.4) |
| Hb (mg/dL) post renal biopsy; median (p25-75) | 9.45 (7.8-10.28) |
| Ht (%) post renal biopsy; median (p25-75) | 28.65 (23.13-31) |
| SBP (mmHg) at the beginning of renal biopsy; median (p25-75) | 139 (131-143) |
| DBP (mmHg) at the beginning of renal biopsy; median (p25-75) | 82.5 (70-90) |
| SBP (mmHg) post renal biopsy; median (p25-75) | 143 (138-147) |
| DBP (mmHg) post renal biopsy; median (p25-75) | 89 (77-94.8) |
SBP: Systolic blood pressure; DBP: Diastolic blood pressure.
Embolization was performed for all patients and was successful in 92% of them [Figure 1]. Only one presented with renal graft loss after embolization. No other associated complications occurred, and additional embolization was not necessary. Median creatinine before embolization was 2.45 (1.55–7.19); 48 h after this procedure, it was 3.05 (p25–75: 1.50–7.18); 3 months later, it had dropped to 1.85 (p25–75: 1.48–3.15) [Figure 2]. The median value of serum creatinine at the last follow-up was 2.58 (p25–75: 1.38–6.23). The graft survival at 1, 6, 12, and 24 months post-biopsy was 91.7%, 83.3%, 71.4%, and 71.4, respectively [Figure 3]. Mortality occurred in one patient but was not related to the procedure.

- Percutaneous embolization. (a) Pre-embolization of the AV fistula (AVF): selective arteriography of the transplanted kidney shows an arterial anastomosis to the external intestinal cavity, with high-grade AVF of the segmental artery of the lower renal pole. (b) Angiographic control post-embolization: complete closure of the AVF was observed, as well as improvement of the parenchymogram
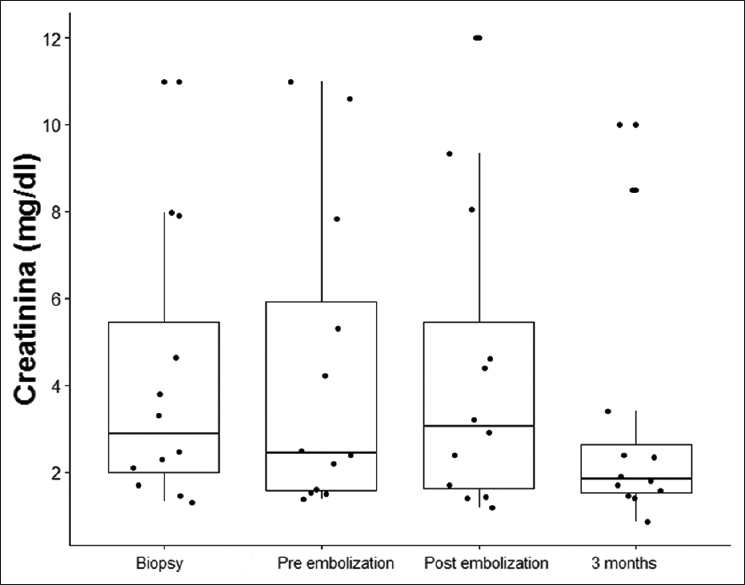
- Serum creatinine values (mg/dL) during follow-up
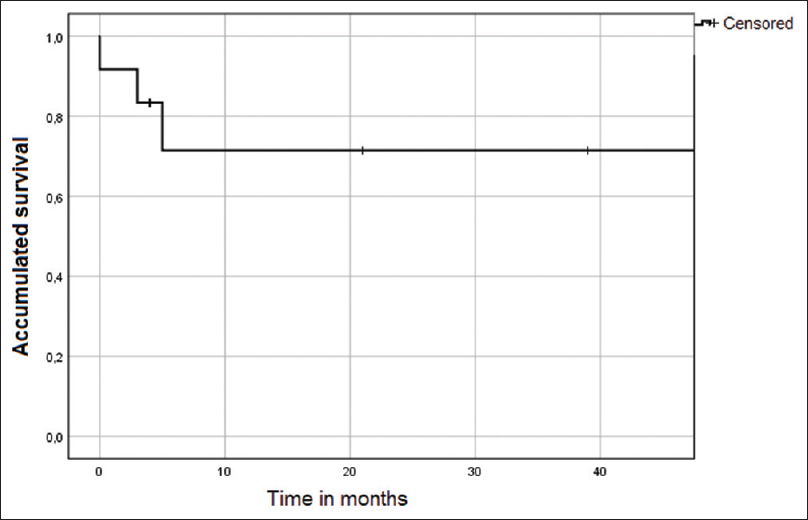
- Survival curve of the renal graft, post-embolization
Discussion
Percutaneous renal graft biopsy is an indispensable procedure in the management of renal transplant patients with graft dysfunction.[79]; However, its invasive nature does not render it a risk-free procedure, as it is one of cause of iatrogenic vascular complications (AVF and pseudoaneurysms).[37] AVF is a complication occurring in 1 to 15% of patients[34]; it is caused by damage of the arterial and venous wall,[10] and diagnosed by graft doppler.[11]
The presence of an AVF in most vessels has little clinical relevance; however, in some cases, it can cause hematuria, hypovolemia, hypertension, and less frequently renal graft dysfunction.[9121314]
Among the available therapeutic options, there is currently no standard therapy.[315] Some groups suggest expectant management, considering that in up to 70% of patients, AVF resolves spontaneously within the first 2 years.[212] Barrios et al. suggest performing ultrasound Doppler to check the graft every week for a month, and then monthly until the AVF is closed.[8] However, some groups are suggesting early intervention; Fossaceca et al. found that endovascular therapy was optimal in symptomatic AVF, or impairment of renal function after kidney biopsy.[3] Concerning endovascular therapy, selective angioembolization is considered the therapy of choice as a safe and effective procedure,[16] which allows occlusion of the AVF without inducing a lesion in the renal parenchyma.
One of the complications of endovascular therapy is the risk of partial infarction or renal ischemia,[2] a complication that did not occur in our patient cohort. Although serum creatinine values after renal embolization significantly increased, this was expected with the contrast medium, or ischemia and inflammation. These levels decreased again when creatinine values were assessed in subsequent controls. Renal function after 2 years of follow-up post-embolization was preserved in 71.4% of patients.
Long-term follow-up of the patients evaluated suggests that embolization is a safe procedure in those with a diagnosis of AVF of the renal graft; this could involve compromise in function, after biopsy of the renal graft, but it allows us to preserve function and avoid its loss in most patients.
Financial support and sponsorship
The study was supported by HPTU, Medellin, Colombia.
Conflicts of interest
There are no conflicts of interest.
References
- Unusual cause of postrenal biopsy anuria in a renal transplant patient. Am J Med Sci. 2011;341:250-2.
- [Google Scholar]
- Post-biopsy arteriovenous fistula in transplant kidney: Treatment with superselective transcatheter embolisation. Eur J Radiol. 2012;81:e721-6.
- [Google Scholar]
- Management of postbiopsy arteriovenous fistulas in transplanted kidneys and effectiveness of endovascular treatment: A single-center experience. Ann Vasc Surg. 2014;28:452-6.
- [Google Scholar]
- Asymptomatic renal pseudoaneurysm after percutaneous renal biopsy. Kidney Res Clin Pract. 2013;32:87-9.
- [Google Scholar]
- Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int. 2004;66:1570-7.
- [Google Scholar]
- Incidence of A-V fistulas after renal biopsy of native and transplanted kidney-Two centers experience. Open Access Maced J Med Sci. 2015;3:241-4.
- [Google Scholar]
- Embolization of postbiopsy and postnephrostomy complications in transplanted kidney: A case report. Transplant Proc. 2008;40:3767-9.
- [Google Scholar]
- Arteriovenous fistulae after renal biopsy: Diagnosis and outcomes using Doppler ultrasound assessment. BMC Nephrol. 2017;18:365.
- [Google Scholar]
- Percutaneous treatment of an arteriovenous fistula and a pseudoaneurysm after a transplanted kidney biopsy. Nefrologia. 2013;33:744-5.
- [Google Scholar]
- Delayed presentation of arteriovenous fistula and pseudoaneurysms in a renal transplant patient 10 years after percutaneous allograft biopsy. Transplant Proc. 2008;40:2444-5.
- [Google Scholar]
- Asymptomatic large extracapsular renal pseudoaneurysm following kidney transplant biopsy. Am J Kidney Dis. 2011;57:175-8.
- [Google Scholar]
- Hemorrhagic shock due to bleeding from an arteriovenous fistula after allograft biopsy in a kidney transplant recipient: A case report. CEN Case Rep. 2018;7:5-8.
- [Google Scholar]
- Analysis of vascular complications after renal transplantation. Transplant Proc. 2011;43:557-61.
- [Google Scholar]
- Arteriovenous fistulas complicating biopsy of renal allografts: Treatment of bleeding with superselective embolization. AJR Am J Roentgenol. 1991;156:507-10.
- [Google Scholar]
- Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol. 2012;7:1591-7.
- [Google Scholar]







