Translate this page into:
Comparative Analysis between Urinary Calprotectin and Serum Creatinine for Early Detection of Intrinsic Acute Kidney Injury
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Acute kidney injury (AKI) is a common and important clinical condition that may lead to chronic kidney disease if it is not diagnosed and treated in its early stages. Urinary calprotectin is a valuable recognized biomarker that can be used to differentiate prerenal and intrinsic AKI. However, till date only a few reports on urine calprotectin measurement in early diagnosis of intrinsic AKI are available. In this study, we compared the sensitivity and specificity of urinary calprotectin with those of serum creatinine in detecting early intrinsic AKI.
Methods:
Over 6 months period (April to October 2018), 81 of 408 patients admitted to the pediatric intensive care unit met the criteria of this cross-sectional study. Their serum creatinine and urinary calprotectin were measured on the first and third day of admission using Jaffe and Elisa radioimmunoassay methods, respectively. The AKI was defined according to the pRIFLE criteria.
Results:
Of the total 81 patients, 67 had the criteria of intrinsic AKI. Of these 62% were female and 38% were male. The mean age of the patients was 22 months. According to data analysis, the area under the curve of ROC of urinary calprotectin on day-1 to detect renal failure is 0.93 with the best cutoff point obtained at 530 ng/mL. The sensitivity, specificity, positive, and negative predictive values of urinary calprotectin levels in diagnosing AKI at this cutoff point are 92.5%, 92.8%, 98.4, and 72.2%, respectively. Besides, urinary calprotectin changes occur much earlier than the rising of serum creatinine.
Conclusion:
Urinary level of calprotectin is a very sensitive biomarker for early diagnosis of intrinsic AKI in children and it can be used in intensive care units or anywhere critically ill children admitted to detect intrinsic AKI. Besides, this study shows that urine calprotectin may be a more sensitive and specific biomarker than serum creatinine in the early phases of intrinsic AKI.
Keywords
Acute kidney injury
calprotectin
intrinsic AKI
serum creatinine
Introduction
Acute kidney injury (AKI) is a clinical syndrome characterized by the inability of the kidneys to excrete nitrogenous and other waste products, maintenance of fluid and electrolytes balance, and acid-base hemostasis. The incidence of AKI appears to increase recently and its etiology is changing from primary parenchymal disease to multifactorial causes.[1] Diagnosis of early phases of AKI and initiation of appropriate therapeutic interventions are of utmost importance to prevent the progression of this critical clinical state and improving its prognosis.[2] Efforts had been made to diagnose and classify different phases of AKI which have largely been dependent upon serum creatinine and urine volume changes.[3] Prolonged prerenal and postrenal types of AKI also, can lead to parenchymal and renal tissue damage. According to KDIGO (Kidney Disease Improving Global Outcome) clinical practice guideline for AKI, it is any situation that can lead to an increase in serum creatinine or decrease of urine output or any combination of them.[4] Nowadays serum creatinine and glomerular filtration rate (GFR) are the main laboratory parameters used to diagnose intrinsic AKI. Imaging studies such as ultrasound examinations greatly help us to detect post-renal causes, but differentiation of pre-renal and intrinsic types may be very difficult. Clinical manifestations of early phases of any type of AKI may be mild and trivial. Decreased urine output has a low sensitivity and specificity in the early stages and GFR has to be decreased at least 30% before the onset of any serum creatinine rise. Besides, there is a relatively long latency between any change in GFR level and increase of serum creatinine e.g., after an abrupt decrease in GFR, it will take at least 1–3 days to serum creatinine raising. Also, the results of any treatment to lower serum creatinine will be lately detectable after GFR returns to normal.[5] Besides, serum urea is not a constant finding and has considerable false positive (e.g., patients on protein-rich diets, any tissue injury, hemorrhages, trauma, and following glucocorticoids therapy) and false negative (e.g. low-protein diet and advanced liver disease) results which hinder its use in detection of AKI.[6] Any biomarker of AKI should have such accuracy and high specificity and sensitivity to help us detect the disease process at its earlier stage, leading to in time appropriate therapeutic interventions preventing further renal tissue damage. There are more than 20 AKI biomarkers already studied.[7] An ideal biomarker should be one that could be easily measured, with no interference with other biologic variables, and be able to detect early phases of kidney damage.[8] The most common biomarkers studied are neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), cystatin-C, L type fatty acid-binding protein (L-FABP), N-acetyl-beta-D glucosaminidase (NAG), netrin-1, vanin-1, and monocyte chemoattractant protein-1 (MCP-1). NGAL and L-FABP are detectable in earlier and KIM-1 and IL-18 in later stages, with higher specificity.[9] In the recent era, there should be a greater impact on clinical practice to detect renal problems much earlier to start treatments when they are more effective.[10] Each of these biomarkers are very valuable, especially in ischemic renal injuries, both experimentally and clinically.[9] Combining different biomarkers is more promising, especially because of the availability of improved methods to validate and quantitate them. The importance of calprotectin among these biomarkers is in its unique properties that make it more practical everywhere. In this study, we compared urinary calprotectin and serum creatinine in AKI cases in PICU, which is more helpful in the diagnosis of AKI in clinical practice.
Methods
All 1–10 years old patients admitted to the pediatric intensive care unit (PICU) ward from April to October 2018 fulfilling the criteria were enrolled in the study. Patients with urinary tract obstruction, urinary tract infection, preexisting primary renal disease, systemic diseases with renal involvement, systemic hypertension, liver disease, and inflammatory bowel disease (IBD) were excluded. The PICU ward was selected in this study owing to the heterogeneity of patients and more probability of AKI occurrence in these children. Besides, renal function tests, urine output, and serum electrolytes were monitored regularly as a routine practice there. Urinary calprotectin, blood urea nitrogen (BUN), and serum creatinine concentrations were measured in the first and third days of admission. Serum creatinine measured with Jaffe method and urine calprotectin was measured by the enzyme-linked immunosorbent assay (ELISA) method using Hitachi 717 machine. The GFR was calculated by the Schwartz formula (k = 0.413). Any patient with GFR less than 90 mL/min/1.73 m2 was regarded as suffering from kidney disease and excluded from the study. Children with intrinsic AKI were classified based on pRIFLE (pediatric Risk, Injury, Failure, Loss, End-stage renal disease) criteria.
This study was a result of a fellowship project and is approved by the research committee of Tehran University of Medical Science. Details of the study were explained to the participants and consent of the patient, their parents, or all of their relatives when applicable was collected before their enrolment in the study. This study had been reviewed also, by the Institutional Ethics Committee of Tehran University of Medical Science (Batch Number 98-02-30-43116) at July/29/2017.
Statistical analysis
Data are presented as mean standard deviation. Receiver-operating characteristic (ROC) curves were formed to determine the accuracy of urinary calprotectin. ROC curve was used to establish the best cutoff value in this study and sensitivity, specificity, positive, and negative predictive value were calculated using this threshold. P values less than 0.05 were regarded as statistically significant. All statistical analyses were done using statistical package for the social sciences (SPSS) Statistics 21.
Results
During the study period, 408 patients were admitted to PICU of whom 81 patients were enrolled in the study. Of these 67 children had intrinsic AKI (mean age 22 months) and 14 cases had non-intrinsic AKI who are not intended for our study [Figure 1]. The mean weight of the patients was 11.8 ± 7.4 kg. About 42 patients (63%) were female. The mean eGFR of the patients was 57 ± 18.5 cc/min/1.73 m2 on the third day. Table 1 demonstrates that the mean urinary calprotectin of intrinsic AKI patients was high even on the first day. On study enrollment, our patients with intrinsic AKI fulfilled the criteria for the pRIFLE category, 24 (35%) of patients for Risk, 37 (55%) Injury, and 6 (9%) Failure. Four of them needed peritoneal dialysis for 5–10 days. Urinary calprotectin was elevated in 62 (92.6%) patients on the first day while it was normal in 5 (7.4%) patients (P-value 0.001). Figure 2 shows the distribution frequency of patients according to their urinary calprotectin level. Ethologic causes of intrinsic AKI are shown in Table 2 along with their mean urinary calprotectin and creatinine level on the third day of admission. There was normal urinary calprotectin in five patients with intrinsic AKI (three cases had gastroenteritis, one with septic shock, and one with cardiogenic shock). Mid and interquartile range (IQR) of calprotectin were 180 and 325 ng/mL ranging between 2 to 1660 ng/mL (P-value 0.001). In respect to the relation between the calprotectin level and AKI, 92.6% of patients with raised calprotectin had intrinsic AKI. The area under the curve of ROC of urinary calprotectin on day-1 to detect renal failure is 0.93 with the best cutoff point obtained at 530 ng/mL. Figure 3 shows the ROC curve for urinary calprotectin level and its cutoff point. The sensitivity, specificity, positive, and negative predictive values of urinary calprotectin levels in diagnosing AKI at this cutoff point are 92.5%, 92.8%, 98.4, and 72.2%, respectively. Table 3 shows sensitivity, specify, positive prognostic value, and negative prognostic value of calprotectin in intrinsic AKI patients with 95% accuracy range.
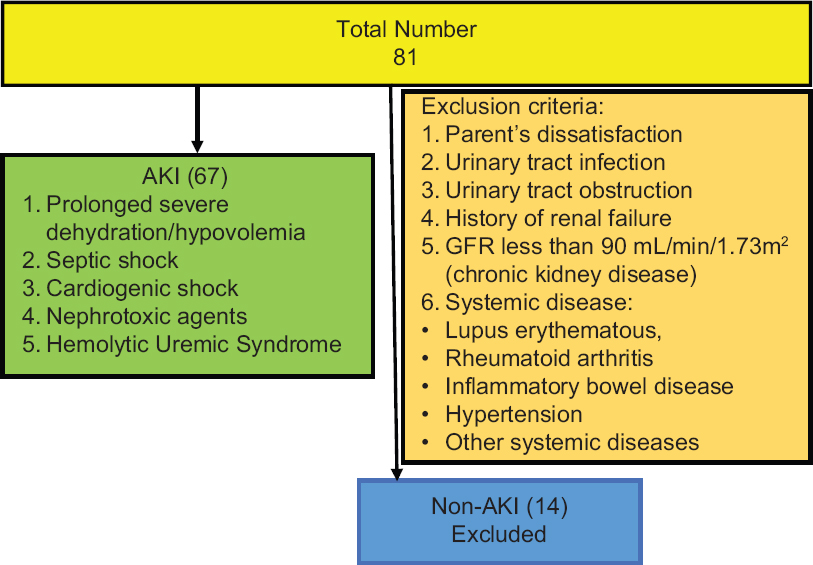
- Flowchart demonstrating enrolled patients
| Factor | 1st day | 3rd day | P-value | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Creatinine | 0.6±0.3 mg/dL | 1.2±0.4 mg/dL | 0.062 | 64% | 59% |
| Calprotectin | 560±390 ng/mL | 962±698 ng/mL | 0.25 | 92.5% | 92.8% |

- Frequency distribution of AKI and non-AKI patients according to calprotectin level
| Cause | Number (percent) | Mean Age (months) | Length of ICU stay (days) | Peritoneal dialysis | Mean Calprotectin (ng/mL) | Mean serum Creatinine level (mg/dL) |
|---|---|---|---|---|---|---|
| Prolonged severe Dehydration/Hypovolemia | 29 (43%) | 5.5 (3-11.5) | 2.5 (1-5.5) | - | 177 | 1.3 (0.9-1.5) |
| Septic Shock | 18 (27%) | 8.6 (5-26) | 5 (3.5-9.5) | - | 432 | 1.5 (1.1-1.8) |
| Cardiogenic Shock | 11 (16%) | 6.5 (3.5-21) | 9.5 (7.0-16) | 2 | 511 | 1.7 (1.2-2.1) |
| Nephrotoxic Agents | 8 (12%) | 14.5 (9.5-36) | 11 (8.5-14) | 1 | 386 | 1.8 (1.6-2.4) |
| Hemolytic Uremic Syndrome | 1 (2%) | 13 | 14 | 1 | 905 | 2.6 (0.9-2.9) |

- ROC curve for urinary calprotectin level
| Value | 95% accuracy rate | |
|---|---|---|
| Sensitivity | 92.5% | 83.6 to 96.7 |
| Specificity | 92.8% | 68.8 to 98.7 |
| Positive prognostic value | 98.4% | 91.5 to 99.7 |
| Negative prognostic value | 72.6% | 49.1 to 87.5 |
Discussion
The outcome of any AKI is critically dependent on its early detection and correction of the causative pathophysiology. Serum creatinine which is used traditionally to detect any deterioration of kidney function is a late marker in AKI due to the lag time between the initiating insult and loss of the kidney function. Serial measuring of serum BUN and creatinine is the standard basis of renal failure diagnosis today. Unfortunately raising of creatinine is a late phenomenon in AKI, while the prognosis of AKI depends on its early diagnosis and starting appropriate treatment.[1112] Calprotectin is an immunomodulatory protein, regarded as an inflammatory factor, and has a protective role in oxidative processes of inflammation.[1314] It is mostly derived from neutrophils and a few amounts are secreted by monocyte and macrophage and is composed of two protein components (S100A8/S100A9) bonding to calcium. Tubular epithelial cells of the collecting system also, secrete S100A8 and S100A9 in response to inflammation; hence calprotectin is detectable in renal tissue injuries. S100A8 and S100A9 monomers are detectable in urine, renal epithelial cells, and blood. It has also been reported in long-lasting urinary tract obstructions, urinary tract infection (secreted from epithelial cells or leukocytes in the urine), rheumatoid arthritis, inflammatory bowel diseases, chronic kidney diseases, and urethra and bladder carcinomas.[291011121314] Detecting calprotectin in urine before its detection or its elevation in serum is much more helpful in clinical practice.[15] In gastrointestinal problems, fecal calprotectin level differentiate between inflammatory bowel disease from functional intestinal problems as this marker is raised only in inflammatory intestinal problems. Therefore, we planned to use urinary calprotectin in comparison with serum BUN and creatinine and GFR to detect its sensitivity and specificity in detecting early stages of AKI in children admitted in our PICU unit. This study shows that urinary calprotectin can be a valuable biomarker to find early renal tissue damage. According to our study, urinary calprotectin level was meaningfully elevated early in children with intrinsic AKI whereas the creatinine level was not notably elevated at the same time (in the first or second day of the disease). According to our study, more than 98% of patients diagnosed as having AKI based on GFR values had elevated calprotectin. It is concluded that the sensitivity and specificity of calprotectin levels to diagnose intrinsic AKI in children is 92.5% and 92.8%, respectively. Also, it's positive and negative prognostic value is 98.4% and 72.2%, respectively. The cut point of 530 has the highest point of sensitivity and specificity in our study. Overall, our results are in accordance with smaller studies performed in the past. The previous studies focused on the value of calprotectin in the differentiation of prerenal from intrinsic AKI.[15] In 2011, Heller et al. performed such a study and resulted that calprotectin has a high diagnostic value in intrinsic AKI and at a 300 ng/mL level has 92.3% sensitivity and 97.1% specificity.[2] Westhoff et al. evaluated other urinary biomarkers to differentiate prerenal and intrinsic pediatric AKIs. They showed that urinary calprotectin outperformed NGAL in discriminating between these entities, whereas KIM-1 failed to do so. The second main finding of this study was that NGAL demonstrated the highest diagnostic performance in the detection of AKI itself, independent of its etiology.[7] More than 98% of patients in our study diagnosed as having intrinsic AKI based on GFR values had also elevated urinary calprotectin. In a similar study, Seibert et al. found that calprotectin and NGAL can be used to differentiate the renal and prerenal forms of AKI and the urinary calprotectin is more sensitive than NGAL in this respect.[1] All previous studies have been done on adult patients and the only one that has been done on children was done by Basiratnia et al. They compared urinary calprotectin level to differentiate functional from structural renal failure. In this study, calprotectin at the level of 230 ng/mL has the highest sensitivity and specificity (95.6% sensitivity and 100% specificity).[15] Furthermore, Seibert evaluated urinary calprotectin to differentiate between prerenal and intrinsic acute renal allograft failure and showed urinary calprotectin as a promising biomarker to differentiate prerenal and intrinsic acute renal allograft failure.[13] The pRIFLE classification system has been suggested as an accurate marker for increased morbidity and mortality in pediatrics[16171819] but we need a more sensitive biomarker than serum creatinine or urine output to diagnose AKI much earlier enabling us to start our treatments in critical time to reduce long-term consequences of AKI. Calprotectin has several exclusive features that make it a valuable clinical marker; it is a local inflammatory marker that is not affected by systemic inflammatory states. Besides, it has high stability at room temperature even for more than 1 week. Also, its measuring method is inexpensive and can be done easily by the ELISA method.[2021] Overall, calprotectin is a stable, sensitive and specific, and economic marker.[22]
Conclusion
The results of our study show that urinary calprotectin has higher sensitivity and specificity than serum creatinine levels for detecting early stages of intrinsic AKI. It's early rising in urine allows us to commence our treatments at earlier stages preventing serious kidney tissue damages in children.
Declaration of patients
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) or their parents has/have given his/her/their consent for his/her/their clinical information's to be reported in the journal. The patients understand that their names and initials will not be published and all due efforts has been made to conceal their identity.
Financial support and sponsorship
There was no financial support or sponsorship in this study.
Conflicts of interest
There is no conflicts of interest in this study.
Acknowledgement
We have to express our appreciation to Seyed Abolhassan Alemohammad M.D. (Chief dermatologist of NIOC hospital, Tehran, Iran) for sharing his pearls of wisdom with us during the course of this research. We are also immensely grateful to him for his comments on an earlier version of the manuscript, although any errors are our own and should not tarnish the reputations of the esteemed professionals.
References
- Calprotectin and neutrophil gelatinase-associated lipocalin in the differentiation of pre-renal and intrinsic acute kidney injury. Acta Physiol (Oxf). 2013;207:700-8.
- [Google Scholar]
- Urinary calprotectin and the distinction between pre-renal and intrinsic acute kidney injury. Clin J Am Soc Nephrol. 2011;6:2347-55.
- [Google Scholar]
- Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 2011;80:545-52.
- [Google Scholar]
- Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246-55.
- [Google Scholar]
- The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557-66.
- [Google Scholar]
- Urinary calprotectin, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin for the prediction of adverse outcome in pediatric acute kidney injury. Eur J Pediatr. 2017;176:745-55.
- [Google Scholar]
- Evaluation of serum and urinary myeloid related protein-14 as a marker for early detection of prostate cancer. J Urol. 2008;180:1309-12.
- [Google Scholar]
- Urinary biomarkers for the differentiation of prerenal and intrinsic pediatric acute kidney injury. Pediatr Nephrol. 2016;31:2353-63.
- [Google Scholar]
- Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121:3425-41.
- [Google Scholar]
- Risk factors of acute renal failure in critically ill patients. Pediatr Crit Care Med. 2007;8:20-35.
- [Google Scholar]
- Urinary calprotectin differentiates between prerenal and intrinsic acute renal allograft failure. Transplantation. 2017;101:387-94.
- [Google Scholar]
- Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:204-12.
- [Google Scholar]
- Urinary calprotectin as a marker to distinguish functional and structural acute kidney injury in pediatric population. Iran J Pediatr. 2017;27:e9727.
- [Google Scholar]
- Urinary biomarkers improve the diagnosis of intrinsic acute kidney injury in coronary care units. Medicine (Baltimore). 2015;94:e1703.
- [Google Scholar]
- Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30:234-54.
- [Google Scholar]
- Acute Kidney Injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:31-8.
- [Google Scholar]
- Monitoring disease activity by stool analyses: From occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58:859-68.
- [Google Scholar]
- Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis. 2009;41:56-66.
- [Google Scholar]
- Proteomic analysis of proteins selectively associated with hydroxyapatite, brushite, and uric acid crystals precipitated from human urine. J Proteome Res. 2010;9:5402-12.
- [Google Scholar]







