Translate this page into:
Outcomes of Kidney Transplantation in the Elderly Recipients
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
In a developing country with a predominantly young population, the valid assumption is directed toward medical care toward the young. However, as medical technology has advanced, quality care has ensured better survival for the elderly population also. The aim of this study was to determine the clinical outcomes in elderly patients undergoing kidney transplantation.
Materials and Methods:
A retrospective analysis of 1150 patients who had undergone live related renal transplantation was done from January 2006 to December 2014. These patients were divided into two groups; Group 1: age >60 years (N = 150), Group 2: age 18–60 years (N = 1000). The clinical outcomes were compared.
Results:
The mean age in Group 1 was 69 ± 7.5 years (SD ± 7.5), and group 2 was 41 ± 8 years. In groups 1 and 2, males were 80% and 82%; death censored graft survival at 5 years was 82% and 87%; patient survival at 5 years was 86% and 94%, respectively. The incidence of biopsy-proven acute rejection was similar in both groups (11.3 vs. 10.2%, P = 0.12). Urinary tract infection was the most common infectious complication. Sepsis was the primary cause of death in both groups.
Conclusion:
In the elderly patients who underwent kidney transplantation, satisfactory graft function, and patient survival were maintained over a period of 60 months. Urinary tract infections were common, and sepsis was the most common cause of death with a surviving allograft. The acute rejection and mortality rates were comparable to the literature published from India so far.
Keywords
Acute rejection
delayed graft function
elderly patients
end-stage kidney disease
kidney transplantation
Introduction
With an increase in average life expectancy, the number of aged individuals with chronic kidney diseases (CKD) is steadily increasing. Currently, there are approximately 420 million people aged >65 years, constituting about 7% of the global population.[1] It is projected that by 2050, there will be >1.5 billion people worldwide aged >65 years, reflecting an increasing number of elderly individuals in both developing and developed countries.[2] Aging is associated with a gradual decline in cellular functions and structural changes in different organs. Coupled with the number of comorbidities, age-related renal function decline increases the risk of CKD in the elderly.[3] Data from the third National Health and Nutrition Examination Survey indicated a much higher prevalence of CKD in persons aged >60 years (39.4%) compared to the persons aged 40–59 years and 20–39 years (12.6 and 8.5%, respectively).[4] The global prevalence of CKD is 13.4%, and 0.1% of them are in stage 5 CKD.[5] The incidence of treated ESKD is highest amongst persons aged >70 years (~3-fold greater than individuals aged 50–59 years).[6]
Kidney transplantation (KT) remains the best available treatment for ESKD. Elderly kidney transplant recipients have shown a more prolonged survival[7]; however, there are limitations after transplantation from the adverse effects of immunosuppressive medications. Several factors need to be considered when evaluating an elderly recipient for transplantation. In addition to recipient comorbidities; donor quality, immunosuppression, dialysis vintage, and the strength of social support networks affect the success of elderly transplantation. Furthermore, the impact of transplantation on the quality of life and mortality was assumed to be not similar to a younger patient.[8] With upgrades in transplant management protocols and an increase in the number of KT in the elderly population, mortality and graft survival outcomes have improved.[7] Studies from India have reported an excellent outcome in elderly KT.[9] We performed this study to further enhance our understanding of clinical outcomes in the elderly undergoing KT.
Materials and Methods
This study is a retrospective analysis, including patients who underwent live related kidney transplantation from January 2006 to December 2014. A total of 1150 patients were enrolled in our study. These patients were divided into two groups as follows, Group 1: age >60 years, Group 2: age 18–60 years. Patients younger than 18 years of age, patients lost to follow-up, and patients undergoing re-transplantation, and combined organ transplantations (with heart or liver) were excluded from the study. Patients were followed up from the date of transplantation to 5 years post-transplantation or until the death of the patient. Patients who were lost to follow up for 60 months were excluded from the study. All patients had undergone age-appropriate cancer screening tests and relevant cardiology assessment. Patients with a normal level of cognitive function were considered for transplants. The clinical details of the patients were retrieved from the hospital medical and electronic health records. The institutional ethical review board of the hospital approved this study.
Immunosuppressive therapy
Immunosuppressive therapy was started 2 days prior to transplantation with tacrolimus 0.15 mg/kg body weight or cyclosporine emulsion 3-5 mg/kg, mycophenolic acid sodium 720 mg twice/day (30–35 mg/kg/day) or azathioprine 2–2.5 mg/kg/day and prednisolone (0.5 mg/kg). The induction protocol was decided as per the immunological status of the patient. On preoperative day 0, patients received induction therapy with either monoclonal antibody (basiliximab) or polyclonal antibodies (ATG) along with methylprednisolone (500 mg). ATG was the primary induction therapy at our center. The dose ATG used was 2-2.5 mg/kg over 1–3 days. In both the groups, the target trough cyclosporine levels ranged from 800 to 1200 ng/mL in the first 3 months, and 500 to 700 ng/mL thereafter. The tacrolimus (tac) trough level was 8–10 ng/mL in the first 3 months, and 5–7 ng/mL thereafter. All patients received 80/400 mg of trimethoprim/sulfamethoxazole daily for an average of 1 year. Following transplantation surgery, we removed urethral catheters on postoperative day 5, irrespective of patient's age; however, the decision of urethral catheter and stent removal was always taken after discussion with transplant surgeons.
Donor evaluation
For donor evaluation, GFR was measured using 99 technetium diethylenetriamine pentaacetic acid (DTPA) scan for differential renal function, and a GFR of more 80 was considered for kidney donation. GFR corrected to age rather than age itself determined acceptability for donation. Renal/urological anatomy was assessed by CT angiography with an excretory urography. All kidney donors underwent hematology and biochemistry profiles; tissue typing, urine protein quantification, chest X-ray, electrocardiography, 2D echocardiography, and screening for infections. Evaluation of the potential medical comorbid conditions such as hypertension, impaired glucose tolerance was done. All donors were screened for standard age- and gender-appropriate screening tests as recommended by national organizations.[10]
Delayed graft function (DGF) was defined as the requirement for dialysis within the first 7 days post-transplantation. A graft biopsy was performed in cases of graft dysfunction. Graft dysfunction was defined as a rise in serum creatinine of >0.3 mg/dL from baseline and/or sustained elevation of serum creatinine of 1.5 mg/dL in the absence of any other etiology. Findings of graft biopsy were then categorized as per the Banff classification of renal allograft pathology (2018).[11] Postoperative complications were graded according to the Clavien-Dindo classification.[12]
The primary outcomes were death censored graft survival and patient survival at 5 years. The secondary outcomes were biopsy-proven acute rejections, surgical complications, new-onset diabetes after transplant (NODAT), infections, and malignancy at 5 years.
Statistical methods
The statistical analysis was performed using the statistical package for social sciences (SPSS, Chicago, Illinois, version 23.0 for Windows). Proportions were compared using the Chi-square or Fisher's exact test wherever applicable. Pearson's Chi-square test and Student's t test were used for categorical and continuous variables, respectively. Graft and patient survival were estimated using the Kaplan–Meier method. A value of P < 0.05 was considered statistically significant.
Results
The donor and recipient profiles and other basic details are shown in Table 1. Initially, 1252 patients were included in the study but later 102 patients were excluded as lost to follow-up. 1150 patients were divided into two groups; Group 1: age >60 years (N = 150), Group 2: age 18–60 years (N = 1000). In group one 46/150 of patients had CAD. During the pre-transplantation assessment, Coronary angiography was performed in all patients of group 1. Asymptomatic cardiac disease was the predominant finding. Out of these 46 patients, 12 patients had significant cardiac disease. Ten patients underwent percutaneous transluminal coronary angioplasty (PTCA), and two patients underwent coronary artery bypass grafting (CABG). Clinical outcome of patients in both study groups are shown in Table 2a. On subgroup analysis of groups 1 and 2, acute cellular rejection and acute humoral rejection were found in 12 (8%) and 3 (2%) patients and 80 (8%), 33 (3.3%) patients, respectively. The incidence of DGF, NODAT, and CNI toxicity was slightly higher in group 1. However, this was not statistically significant. [Table 2a] On multivariate analysis, the age of the recipient was not found to be associated with DGF, BPAR, and NODAT and CNI toxicity. Surgical complications, which were higher in group 1 as compared to group 2 are shown in Table 2b. We did not find any significant difference in the mean duration of retention of urethral catheters in both groups. Surgical complications were classified according to Clavien–Dindo grading of surgical complications during the first 30 days post-transplant days in group 1. Thirty percent of the complications were Grade I, 22% Grade II, 17% Grade IIIa, 8% Grade IIIb, 1.34% Grade IVa, 0.7% Grade IVb, and nil in Grade V. Urinary tract infections (UTI) followed by pneumonia were amongst the commonest infectious complication in both the groups. Figure 1 depicts the infectious complications in group 1. No difference was found in the detection of cytomegalovirus (CMV) infection, BK virus nephropathy, and pneumocystis jirovecii infection in either of the groups. Malignancy was diagnosed in 1 (0.6%) and 2 (0.2%) patients in groups 1 and 2, respectively.
| Characteristics | Group 1 (n=150) n (%) | Group 2 (n=1000) n (%) | P |
|---|---|---|---|
| Age, years ± (SD) | 69±7.5 | 41±8 | 0.02 |
| Male gender, n (%) | 120 (80) | 820 (82) | - |
| ESRD cause, n (%) | |||
| Diabetes mellitus | 79 (52.3) | 414 (41.4) | NS |
| Chronic Glomerulonephritis | 30 (20.33) | 300 (30) | - |
| Chronic interstitial nephritis | 22 (14.7) | 228 (22.8) | - |
| Hypertensive nephrosclerosis | 12 (8.33%) | 10 (1) | <0.05 |
| Undetermined | 7 (4.37) | 48 (4.8) | - |
| Co-morbidities | |||
| Diabetes | 82 (55) | 452 (45.2) | NS |
| Hypertension | 127 (85) | 900 (90) | - |
| Coronary artery disease | 46 (31) | 250 (25) | - |
| Peripheral artery disease | 9 (6) | 30 (3) | - |
| Chronic obstructive pulmonary disease | 12 (8) | 40 (4) | - |
| ABO-incompatible | 25 (17) | 250 (25) | NS |
| Pre-emptive Transplant | 20 (13.5) | 160 (16) | - |
| Median time in dialysis, months | 12 (2-36) | 8 (1-30) | NS |
| Hemodialysis | 115 (88) | 750 (89.2) | - |
| Peritoneal dialysis | 15 (12) | 90 (10.7) | - |
| HLA Mismatch | |||
| 1 | 25 (16.7) | 208 (20.8) | - |
| 2 | 23 (15.7) | 170 (17) | - |
| 3 | 31 (20.7) | 180 (18) | - |
| 4 | 21 (14.7) | 140 (14) | - |
| 5 | 32 (22.0) | 172 (17.2) | - |
| 6 | 19 (13.3) | 130 (13) | - |
| Donor characteristics | |||
| Age | |||
| < 50 years | 57 (38) | 790 (79) | <0.05 |
| 50-60 years | 69 (45.8) | 200 (20) | 0.02 |
| 60-70 years | 24 (16.2) | 10 (1) | 0.004 |
| Living donors (%) | 150 (100) | 1000 (100) | - |
| Relationship with recipient, n (%) | |||
| Siblings | 56 (37.5) | 144 (14.4) | <0.05 |
| Spouse | 54 (35.5) | 390 (39) | NS |
| Children | 3 (2) | 3 (0.3) | - |
| Other relatives | 37 (25) | 131 (13.1) | - |
| Parents | - | 332 (33.2) | 0.002 |
| Induction agent | 123 (82) | 840 (84) | - |
| Basiliximab | 34 (27.8) | 200 (23.8) | - |
| Anti- thymocyte globulin | 89 (72.2) | 640 (76.2) | - |
| Initial immunosuppression | |||
| Steroid +Mycophenolate Mofetil + tacrolimus | 142 (95.2) | 978 (97.8) | NS |
| Steroid + Mycophenolate Mofetil + Cyclosporine | 6 (3.4) | 12 (1.2) | NS |
| Steroid + Azathioprine + Tacrolimus | 2 (1.4) | 10 (1) | NS |
| Group 1 n=150 | Group 2 n=1000 | P | |
|---|---|---|---|
| Complications following kidney transplantation, n (%) | |||
| Delayed graft function | 22 (15) | 100 (10) | 0.2 |
| Biopsy proven acute rejection | 15 (10.2) | 113 (11.3) | 0.12 |
| CNI toxicity | 24 (16) | 124 (12.4) | 0.07 |
| Surgical complications | 20 (13) | 60 (6) | <0.05 |
| NODAT | 29 (19.4) | 150 (15) | - |
| Urinary tract infections | 59 (39.5) | 280 (28) | 0.03 |
| Cytomegalovirus infection | 16 (8) | 70 (7) | - |
| B K virus nephropathy | 18 (12.4) | 98 (9.8) | - |
| Pneumocystis jirovecii infection | 3 (2) | 10 (1) | - |
| Pneumonia | 34 (22.5) | 12 (1.2) | <0.05 |
| Malignancy | 1 (0.6) | 2 (0.2) | - |
| Group 1 n=20 (13%) | Group 2 n=60 (6%) | P | |
|---|---|---|---|
| Surgical Complications, n | |||
| Complications | <0.05 | ||
| Hemorrhages | 9 | 41 | |
| Lymphoceles | 7 | 11 | |
| Urethral stenosis | 4 | 7 | |
| Renal artery thrombosis | 0 | 1 |
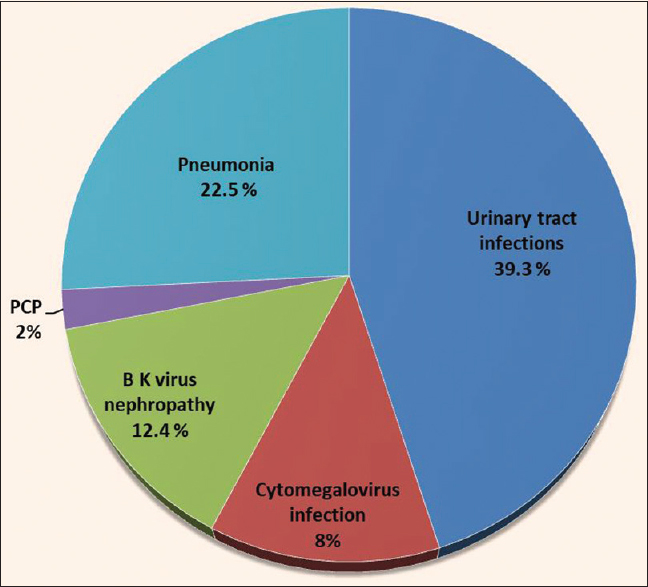
- Infectious complications in elderly population post kidney transplantation
Patient and graft survival
Patient and graft survival in both the groups is shown in Table 3. The causes of graft loss, excluding recipient death in group 1 and group 2 were fibrosis/atrophy (IF/TA) (32% vs 28%), recurrent disease, (10% vs 15.7%), rejection (16% vs 18%), BK virus nephropathy (9% vs 5%), and other causes (33% vs 33.3%). No graft was lost due to primary graft non-function (absence of graft function immediately posttransplant) and surgical complications in both the groups. No statistically significant correlation was found between graft loss and patient age. Figure 2 is showing Kaplan–Meier of death censored graft survival.
| 12 months | 24 months | 36 months | 48 months | 60 months | |
|---|---|---|---|---|---|
| Death censored graft survival, n (%) | |||||
| Group 1 | 146 (96.4) | 135 (90) | 130 (86.6) | 125 (83.3) | 123 (82) |
| Group 2 | 990 (99) | 977 (97) | 950 (95) | 890 (89) | 867 (87) |
| Patient survival, n (%) | |||||
| Group 1 | 147 (98) | 142 (94.6) | 138 (92) | 132 (88) | 129 (86) |
| Group 2 | 991 (99.1) | 982 (98.2) | 976 (97.6) | 954 (95.4) | 940 (94) |

- Kaplan–Meier––death censored graft survival
The overall analysis of survival rates amongst both the groups showed no difference at 1 year (98% vs. 99.1%). However, the 5 years survival rate declined in the elderly population in group 1 versus group 2 (86% vs. 94%) (P = 0.06) [Table 3]. During the follow-up period, twenty-one patients died primarily due to sepsis (56%), cardiovascular accidents (34%), cerebrovascular accidents (5%), and others (5%). None of the patients died because of malignancy. The causes of death in both groups are shown in [Table 4]. We did not observe any statistically significant differences between the recipients treated with ATG or Basiliximab in terms of patient and graft survival, infections and acute rejection.
| Group 1 | Group 2 | |
|---|---|---|
| Sepsis | 12 (56) | 16 (54) |
| Cardiovascular accidents | 7 (34) | 9 (30) |
| Cerebrovascular accidents | 1 (5) | 1 (3) |
| Others | 1 (5) | 4 (13) |
In multivariate analysis [Table 5], diabetes mellitus was the only factor that was independently associated with an increased risk of mortality (HR 2.28; 95% CI 1.53 to 3.51; P < 0.01). Other variables such as other etiology of ESKD, age more than 60 years, gender, the modality of dialysis before transplant, dialysis vintage, and type of induction agent (basiliximab or antithymoglobulin) were not found to be associated with higher patient mortality.
| Multivariate Cox Proportional Hazard Ratio Analysis For Patients Mortality | |||
|---|---|---|---|
| Variables | HR | 95% confidence interval | P |
| Diabetes mellitus | 2.28 | 1.53 to 3.51 | <0.01 |
| Etiology of ESKD (excluding diabetes mellitus) | 1.22 | 1.028-1.62 | 0.2 |
| Age >60 years | 1.32 | 1.06-2.07 | 0.06 |
| Male Gender | 0.72 | 0.82-1.79 | 0.24 |
| Modality of dialysis before transplant | 1.32 | 0.94-2.04 | NS |
| Dialysis vintage | 2.034 | 1.063-3.61 | 0.37 |
| Antithymoglobulin | 1.61 | 1.18-3.86 | 0.11 |
Impact of donor age
On log hazard ratio analysis in group 1, with increased donor age (>60 years) the odds of DGF were increased by OR 1.19 (95% CI: 1.11–1.21), and death censored graft failure was increased by OR 1.59 (95% CI: 1.32, 1.71). No risk of acute rejection and difference in patient survival was detected with elderly donors. Rates of postoperative complications were not significantly different between the donors of age more than 60 years and less than 60 years.
Discussion
Hemodialysis, peritoneal dialysis, and KT are the well-established options for the management of ESKD. In elderly patients with ESKD, choosing an appropriate therapeutic option is necessary to improve the mortality outcomes and quality of life. Among these, KT is considered the most optimal modality of renal replacement therapy. Contrary to previous beliefs that the elderly should be managed with hemodialysis in the view of their short lifespan and risk of death with a functioning draft, KT is found to be of significant survival benefit in the elderly population.[7] Among the elderly patients on dialysis, transplantation is associated with a 41% lower risk of death compared with the survival of comparable candidates on the waiting list.[13] In our study, we showed a patient survival rate of 98% and 86% at one and 5 years, which was comparable with the survival rate in another group (18–60 years). A study from Kute et al. on 103 KT patients reported patient survival of 93% and 83.3%, 1 year and 5-year, respectively.[9] contrary to that, in another study by Nial et al.,[14] elderly recipients had higher mortality at 1-year post-transplant than younger population (6.8% vs. 2.1%; P = 0.03). We found only diabetes mellitus as an etiology of kidney failure an independent risk factors for decreased patient survival. However, Paula et al. showed a significant co-relation of age and deceased donor apart from diabetes mellitus with reduced patient survival.[15]
In the above-mentioned study by Kute et al.[9] death-censored graft survival was 97.3% and 92.5% at one, and 5 years, respectively, we found comparable results at 1 year (96.4%), but in our study 5 years, death-censored graft survival declined to 82%. Possibly due to the inclusion of patients of higher age group (>60 years) than their study (>55 years) also 16.2% of kidney donors in the elderly group were of age >60 years in our study. Our results were consistent with the findings of another study.[16] The use of kidneys from older donors is associated with an increased risk of transplant failure. In an analysis by Cecka et al. based upon data of the UNOS Scientific Renal Transplant Registry, the relative risk of death-censored graft failure among recipients of all ages who received a kidney from a donor ≥55 years almost doubled as compared to donors aged 15–24 years. Patients of 65 years and older receiving a kidney from a donor ≥55 years had a relative risk of graft loss that was 3.6 times higher than patients between 18 and 34 receiving kidneys from donors between 15 and 24 years.[17]
Previously published data revealed reduced chances of acute rejection but a higher incidence of DGF in elderly recipients as compared to young patients.[18] Another study also showed a higher incidence of acute rejection (28.7%) in the younger recipient than in the elderly cohort (15.9%).[19] We reported a similar incidence of BPAR in both groups (11.3 vs. 10.2%, P = 0.12), but the occurrence of DGF was slightly higher in the elderly group. Reduction of naive T cells, heightened sensitivity to immunosuppressive therapy, and an increase in the number of T suppressor cells are the possible mechanism responsible for lower chances of acute rejection in elderly recipients. An impaired co-stimulatory pathway, the most critical part of allorecognition for T-cell activation is also an important attributable factor for less immunogenicity.[2021] In elderly group out of 150 patients, 123 (82%) patients received induction therapy still we got 17 cases of acute rejection. Earlier it has been suggested that less aggressive induction therapy may be warranted in the elderly transplant recipients. In our study, we included only live related transplants, but worldwide increased utilization of organs from expanded criteria donor has introduced an added layer of complexity in the early post-transplant management of elderly transplant recipients because most kidney allocation strategies preferentially allocate organs from higher-risk donors to elderly recipients.[22] Of note, increasing age of both recipients and donors predisposes for surgical complications. We found more surgical complications in elderly cohorts than in the age group 18–60 years (13% vs 6%, P < 0.05). Hemorrhage was the most frequent complication. Our results were consistent with the results of other studies. An analysis done by Bental et al.[23] showed an increased risk of surgical complications (47%) in elderly population when compared to younger recipients (28%). Pinto et al.[24] also reported an association of older age with surgical complications (P = 0.023).
Sepsis (11 patients) followed by cardiovascular (7 patients) and cerebrovascular accidents (2 patients) were the major causes of death in the elderly population. Analysis by Kriesche et al. utilizing the United States Renal Data System database also showed an exponential rise in mortality from infections in older KT recipients as compared with younger patients. The mortality risk from infections increases in elderly recipients to a much higher degree than the waiting list of patients.[25] The two peculiar concerns in elderly patients are diverse pharmacokinetics of immunosuppressive medications, in addition to age-related immunosenescence increase their vulnerability to opportunistic infections.[2627] Factors not related to old age such as poverty, unsanitary living conditions, tropical climate, financial constraints, and induction therapy with r- ATG are some other crucial reasons for the higher incidence of infection rate in transplant settings, especially in developing countries.[28293031]
This study is one of the few Indian studies comparing outcomes of kidney transplantation in the elderly (>60 years) versus non-elderly (18–60 years) recipients. Optimal patient survival rates, comparable rejection rate, and DGF occurrence indicate that KT is definitely a viable option to prolong life in elderly patients with ESKD. There are some limitations to the study. It is a single-center study with a follow up of 5 years, as long term follow up might help us in predicting the long term outcomes of the graft. Being a retrospective analysis, the reliability data available from medical records have certain limitations. The larger sample size and inclusion of deceased donors might get more clarity regarding the graft survival and long term survival of the elderly population. There could be a possibility of case selection bias as we did not find many patients with multiple co-morbidities who underwent transplantation. Some other aspects, like the factors predisposing to UTI, assessment of the quality of life and emotional status of the recipients, were not evaluated in our study.
Conclusion
Kidney transplants with patient-tailored approaches can help us achieve better graft function and patient survival. Results observed in our setting are promising with lower rates of rejection and mortality. Diabetes mellitus was a major determining factor responsible for higher mortality. Infectious complications need to be watched for as they are the most frequent cause of death in the post-transplant period. With our experience, we observe a need for large, prospective, long-duration studies with a control group to substantiate the benefits of KT in the elderly.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Chronic kidney disease and end-stage renal disease in the elderly population: Current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17:293-301.
- [Google Scholar]
- Prevalence of chronic kidney disease and decreased kidney function in the adult US population. Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1-12.
- [Google Scholar]
- Global prevalence of chronic kidney disease-A systematic review and meta-analysis? PLoS One. 2016;11:e0158765. doi: 10.1371/journal.pone. 0158765
- [Google Scholar]
- Incidence, management, and outcomes of end-stage renal disease in the elderly. Curr Opin Nephrol Hypertens. 2009;18:252-7.
- [Google Scholar]
- Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc. 2014;62:2235-42.
- [Google Scholar]
- Optimization of treatment modality in elderly end-stage renal disease population: Peritoneal dialysis versus transplant. Indian J Nephrol. 2018;28:433-40.
- [Google Scholar]
- Renal transplantation in elderly patients older than 70 years of age: Results from the scientific registry of transplant recipients. Transplantation. 2007;83:1069-74.
- [Google Scholar]
- Renal function as a predictor of long-term graft survival in renal transplant patients. Nephrol Dial Transplant. 2003;18(Suppl 1):i3-6.
- [Google Scholar]
- Outcome of live and deceased donor renal transplantation in patients aged≥55 years: A single-center experience. Indian J Nephrol. 2014;24:9-14.
- [Google Scholar]
- KDIGO Clinical practice guideline on the evaluation and care of living kidney donors? Transplantation. 2017;101(8 Suppl 1):S7-105. doi: 10.1097/TP. 0000000000001769
- [Google Scholar]
- A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation. 2018;102:1795-814.
- [Google Scholar]
- Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13.
- [Google Scholar]
- Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-30.
- [Google Scholar]
- Outcomes following renal transplantation in older people: A retrospective cohort study. BMC Geriatr. 2013;13:79.
- [Google Scholar]
- Long-term outcomes of elderly kidney transplant recipients. J Bras Nefrol. 2015;37:212-20.
- [Google Scholar]
- Predicting clinical outcome in the elderly renal transplant recipient. Kidney Int. 2000;57:2144-50.
- [Google Scholar]
- The UNOS scientific renal transplant registry. Clin Transpl 1999:1-21. PMID: 11512303) (Volume not available)
- [Google Scholar]
- Renal registry committee.Transplantation versus haemodialysis in elderly patients. Nephrol Dial Transplant. 1997;12:261-4.
- [Google Scholar]
- Renal transplantation in the elderly: South Indian experience. Int Urol Nephrol. 2011;43:265-7.
- [Google Scholar]
- Early lymphocyte activation in elderly humans: Impaired T and T-dependant B-cell responses. Exp Gerontol. 1999;34:217-29.
- [Google Scholar]
- Induction of allograft tolerance through costimulatory blockade: First selection of drugs in vitro. Transpl Immunol. 2003;11:215-22.
- [Google Scholar]
- Induction immunosuppressive therapy in the elderly kidney transplant recipient in the United States. Clin J Am Soc Nephrol. 2011;6:1168-78.
- [Google Scholar]
- Renal transplantation in the elderly: Surgical complications and outcome with special emphasis on the Eurotransplant Senior Programme. Nephrol Dial Transplant. 2008;23:2043-51.
- [Google Scholar]
- Surgical complications in early post-transplant kidney recipients. Transplant Proc. 2017;49:821-3.
- [Google Scholar]
- Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int. 2001;59:1539-43.
- [Google Scholar]
- Global scientific vision with local vigilance: Renal transplantation in developing countries? Nephrourol Mon. 2014;7:e22653. doi: 10.5812/numonthly. 22653
- [Google Scholar]
- Developing world perspective of post transplant tuberculosis: Morbidity, mortality and cost implications. Transplant Proc. 2001;33:1787-8.
- [Google Scholar]
- Opportunistic infections occurring in renal transplant recipients in tropical countries. Indian J Transplant. 2019;13:110-4.
- [Google Scholar]







