Translate this page into:
Microalbuminuria and Urinary Neutrophil Gelatinase-associated Lipocalin (uNGAL) in Human Immunodeficiency Virus Infected Children
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Renal dysfunction and progression to end stage renal disease is well known in human immunodeficiency virus (HIV) infection. We studied the role of microalbuminuria and urinary NGAL levels in children with HIV infection for the prediction of renal dysfunction.
Design and Methods:
A cross-sectional study was carried out and 60 HIV infected children, aged (18 months to 15 years) were screened for microalbuminuria by nephelometry and for uNGAL by ELISA. Thirty healthy children were screened for uNGAL for normative data in Indian children.
Results:
The prevalence of microalbuminuria in studied population was 3.3%. The mean uNGAL and uNGAL/creatinine in study population was higher than controls (26.94 ± 93.12 ng/ml vs. 88.94 ± 345.20 mcg/g, and 15.53 ± 37.52 ng/ml vs. 30.12 ± 78.66 ng/ml; P = 0.003, P = 0.002). Children with lower CD4 counts had significant higher mean Albumin Creatinine Ratio (ACR) and mean uNGAL; P = 0.03, P = 0.01.
Conclusions:
uNGAL and urine microalbumin are useful biomarkers of early tubular and glomerular injury in children with HIV infection.
Keywords
Children
HIV
microalbuminuria
NGAL
renal dysfunction
Introduction
Accessible and affordable anti-retroviral therapy has changed the outcome of human immunodeficiency virus (HIV) infection from a potentially lethal disease to a chronic manageable infection. Heavy proteinuria and its rapid progression to end stage renal disease (ESRD) have been well recognized in HIV infected individuals. Renal disease was estimated to be the fourth leading cause of death by EuroSIDA study in the year 2009.[1] Renal manifestations can vary from glomerular diseases like HIV associated nephropathy (HIVAN) to interstitial diseases and medication related complications. The pathogenesis of renal damage depends on the interplay between immune and genetic factors of both virus and the host.[2] Early screening and antiretroviral therapy (ART) have led to improved outcomes in HIV infected individuals with kidney disease. There is a need for a robust biomarker which can identify renal damage early in the course of the disease. Microalbuminuria is an established early marker of subclinical renal disease and glomerular pathology.[3] Studies among adults and children worldwide have shown significantly increased levels of micro albuminuria ranging from 12% to 20% in HIV infected patients.[4567] Neutrophil gelatinase-associated lipocalin (NGAL) is markedly upregulated in renal tubules in response to epithelial damage. Hence, urinary neutrophil gelatinase-associated lipocalin (uNGAL) is a useful biomarker for early recognition of tubular injury.[8] Recent studies among HIV infected people with renal disease have shown high urinary uNGAL levels to be correlating with HIVAN.[910] There is paucity of reports regarding renal manifestation and uNGAL in children with HIV, especially from the Indian subcontinent. This manuscript presents urine microalbumin and uNGAL in children with HIV infection.
Patients and Methods
This study was conducted in Pediatric Immunodeficiency Clinic at Advanced Pediatrics Centre, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India from July 2012 to June 2013. The institute is federally funded, not for profit, tertiary care centre in North-West India catering to children with HIV. It is a recognized Centre for Excellence for HIV care by the National AIDS Control Organization. A total of 734 children living with HIV infection were registered till June 2013 and a convenient sample of 60 children who fulfilled the inclusion criteria from this cohort were enrolled during the study period [Figure 1]. The study population was divided into three groups with 20 children each: Group 1: Pre-ART defined as ART naïve with stable CD4 counts not warranting initiation of ART as per national guidelines.[11] [NACO 2006]; Group 2: ART naïve children with severe immunosuppression warranting initiation of ART; and Group 3: Children on ART for more than 1 year (n = 20). Children with congenital renal malformations, past history of any renal disease, or active urinary tract infection (UTI) were excluded. At enrollment, information related to demography, clinical profile, and examination findings were noted. Routine evaluation of viral loads was not a standard of care under the NACO during the study period. Written informed consent was taken from parents or primary caregiver in all the subjects. Assent was taken from the children if aged more than 8 years and were disclosed of their HIV status. Thirty consecutive healthy children of age between 3 and12 years were enrolled from immunization clinics with informed consents from parents or caregivers as control group. Urinary samples were collected from control population for uNGAL estimation. The details of recruitment process of children with HIV are depicted in [Figure 1].
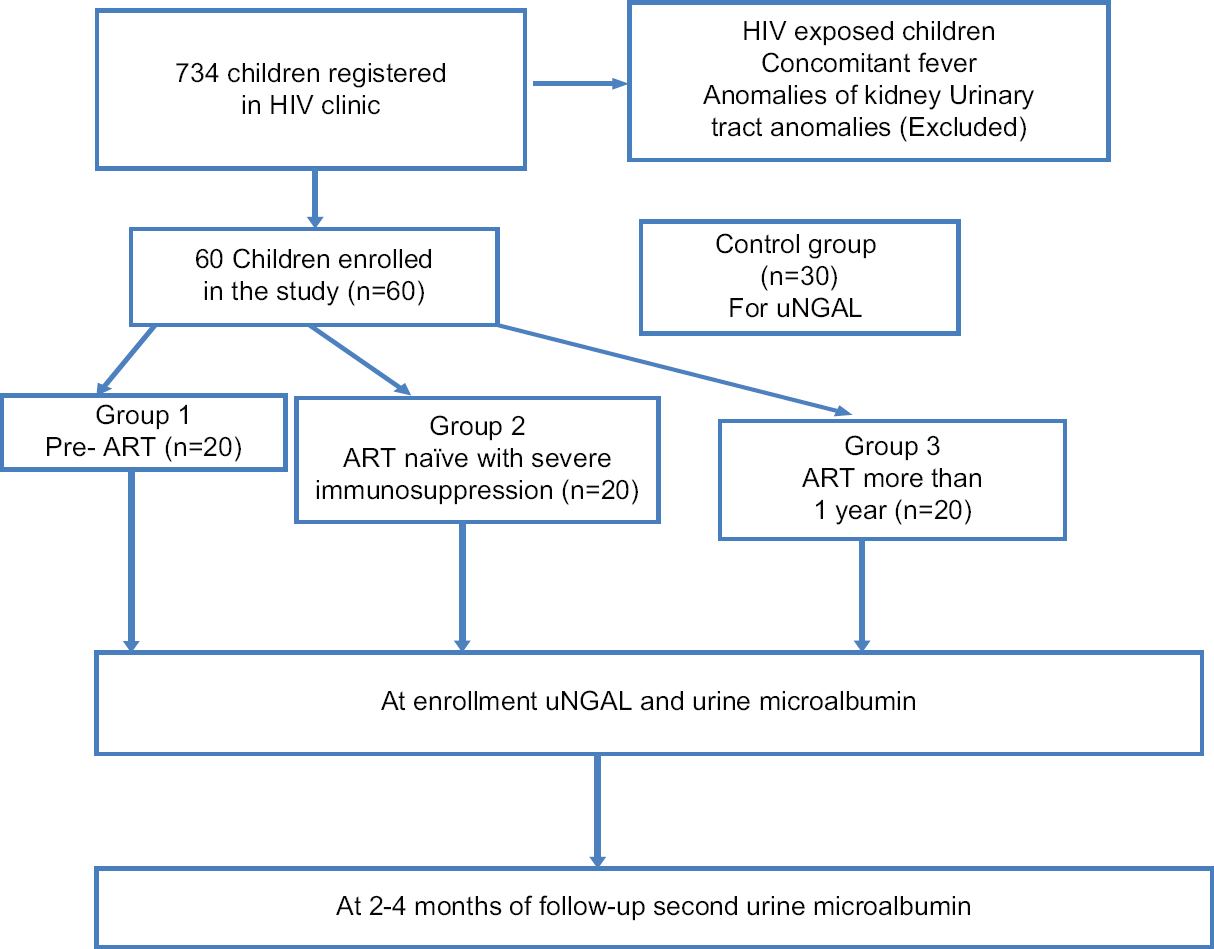
- Recruitment of children with HIV into different groups
Laboratory methods
First morning urine sample were collected on two separate occasions, first at the time of enrollment and second after 2--4 months and tested for albumin by dipstick. Urine culture and sensitivity was done to rule out active UTI. Urine microalbumin was measured using Minineph Human microalbumin kit (ZK302), Minineph TM, Birmingham, United Kingdom by nephelometry. Urine creatinine was estimated by colorimetry, applying the Larsen modification of Jaffe reaction using SIEMENS CREA, Siemens healthcare, Munich on Dimension R clinical chemistry system. Microalbuminuria was defined as albumin/creatinine ratio (ACR) of 2.5-25 mg/mmol in males and 3.5-25 mg/mmol in females in urine specimen in accordance with ISPAD guidelines.[12] ACR was calculated for each sample and twice for each patient. For uNGAL, urine samples obtained at the time of enrolment were tested by ELISA using Human ELISA Kit (KIT 037) by Bioporto Diagnostics Gentofte, Denmark. The uNGAL was further corrected for urinary creatinine excretion by calculation of uNGAL/creatinine ratio. As normative data in uNGAL in Indian pediatric population was not available, the results were compared against control population enrolled in the study. Estimated Glomerular filtration rate (eGFR) was calculated as by the Schwartz formula[13] and the cut off taken for low eGFR was 90 ml/1.73 m2/min.[14] Ethical approval was obtained from the Institutional Ethics Committee.
Statistical analysis
All filled questionnaires were coded before entering into the computer using Statistical Package for Social Sciences (SPSS) version 20 and analyzed. Frequency distribution and two-way tables were used to summarize the data and association between independent and dependent categorical variables were determined by Chi-square X2 and Fisher's exact tests. Student's t-test was used to determine association between means. Non-parametric tests including Mann-Whitney U and Kruskal-Wallis were used to determine association between 2 or >2 groups. A P value of <0.05 was considered statistically significant. For correlation studies of non-parametric variables, Spearman's rho correlation was used. A value of 0.3-1 was considered significant.
Results
The baseline characteristics of the study population are shown in Table 1. There was significant proteinuria in 1 child. Blood urea and serum creatinine levels were normal in all children except 1 child. Fourteen children (23.3%) had low eGFR and eight of them were in second group. The ACR values were higher in second group, in both the samples and the mean. This was statistically significant with P value of 0.006 (ACR1), 0.035 (ACR 2), and 0.034 (mean ACR), respectively. Severe immunosuppressed second group had a higher uNGAL mean value and this finding was statistically significant with a P value of 0.010. Corrected uNGAL for urine creatinine excretion also showed similar findings with the severe immunosuppressed group having higher values, which was statistically significant. On comparison with the control group, uNGAL and corrected uNGAL for creatinine were found to be higher in study group with statistical significance. The respective values were 26.94 ± 93.12 ng/ml, 88.94 ± 345.20 mcg/g in the study population and 15.53 ± 37.52 ng/ml, 30.12 ± 78.66 mcg/g in the control group. [Table 2]. Similar significant difference was noted between the control group and second group (P = 0.001, P = 0.001), with values of 60.78 ± 157.72 ng/ml and 222.79 ± 582.29 mcg/g in the second group, while that in control group 15.53 ± 37.52 ng/ml and 30.12 ± 78.66 mcg/g, respectively, for uNGAL and corrected uNGAL for creatinine. The correlation of uNGAL with the severity of immunosuppression was studied which showed a definite negative correlation coefficient of -0.310 with CD4 count and -0.532 with the CD4%, respectively.
| Characteristic | ART* naïve children with stable CD4 counts for >5 years n=20 (%) | ART naïve children with severe immunosuppression n=20 (%) | Children on ART for >1 year n=20 (%) | Total n=60 (%) | P |
|---|---|---|---|---|---|
| Mean age (years) | 10.25±2.84 | 7.67±4.35 | 10.39±3.54 | 9.48±3.79 | |
| Male | 10 (50) | 15 (75) | 14 (70) | 39 (65) | |
| Duration of disease (years) | 10.05±2.93 | 7.22±4.19 | 10.25±3.58 | 9.28±3.75 | 0.028 |
| n=19 | n=16 | n=19 | n=54 | ||
| Asymptomatic | 10 | 2 | 5 | 17 (28) | 0.018 |
| Failure to thrive | 4 | 16 (80) | 8 | 28 (46) | 0.008 |
| Pallor | 7 (35) | 17 (85) | 3 (15) | 26 (43) | <0.001 |
| Lymphadenopathy | 14 (20) | 15 (75) | 2 (10) | 21 (35) | |
| Stage 1 CKD | 14 (70) | 4 (20) | 17 (85) | 35 (58) | <0.001 |
| Stage 2 CKD | 6 (30) | 8 (40) | 1 (5) | 15 (25) | |
| Stage 3 CKD | 7 (35) | 1 (5) | 8 (13) | ||
| Stage 4 CKD | 1 (5) | 1 (5) | 2 (3) | ||
| Mean CD4 count (mm3) | 772.45±316.72 | 510.95±506.89 | 776.65±418.57 | 686.68±432.63 | |
| Mean CD4% | 21.65±7.48 | 12.81±6.66 (n=17) | 25.42±9.07 | 20.05±9.27 | <0.001 |
| Spot urine protein | |||||
| >2+ | 1 | 1 (1.5) | |||
| Blood urea (mg/dl) | 19.85±5.57 | 22.35±20.30 | 20.90±6.63 | 21.03±12.57 | |
| Serum creatinine (mg/dl) | 0.49±0.16 | 0.59±0.49 | 0.48±0.14 | 0.52±0.31 | |
| Low eGFR | 4 | 8 (40) | 2 | 14 (23.3) | |
| Mean eGFR (ml/1.73 m2/min) | 134.83±52.27 | 105.65±54.59 | 130.32±44.47 | 123.44±51.48 | 0.028 |
| ACR1 (mg/mmol) | 0.31±0.29 | 1.45±2.9 | 0.45±0.40 | 0.74±1.76 | 0.006 |
| ACR2 (mg/mmol) | 0.26±0.14 | 0.92±1.65 | 0.39±0.23 | 0.52±0.99 | 0.035 |
| Mean ACR (mg/mmol) | 0.35±0.26 | 1.19±2.25 | 0.43±0.27 | 0.65±1.35 | 0.034 |
| Mean uNGAL (ng/ml) | 10.16±8.32 | 60.78±157.72 | 9.87±13.25 | 26.94±93.12 | 0.010 |
| Mean NGAL/creatinine (mcg/g) | 18.89±17.34 | 222.79±582.29 | 25.14±48.54 | 88.94±345.20 | 0.006 |
| Persistent microalbuminuria n (%) | 0 | 2 (3.3%) | 0 | 3.3% | 0.0001 |
*ART: Anti retroviral therapy; GFR: Glomerular filtration rate; ACR: Albumin creatinine ratio; uNGAL: Urinary neutrophil gelatinase associated lipocalin; CKD: Chronic kidney disease
| Variable | Study population n=60 | Normal population n=30 | P |
|---|---|---|---|
| uNGAL* (ng/ml) | 26.94±93.12 | 15.53±37.52 | 0.003 |
| uNGAL/creatinine (mcg/g) | 88.94±345.20 | 30.12±78.66 | 0.002 |
*uNGAL: Urinary neutrophil gelatinase associated lipocalin
Discussion
Renal involvement in HIV infected population encompasses chronic glomerular disorders like HIVAN, HIV immune complex kidney disease (HIVICK), thrombotic microangiopathies, disorders of proximal tubular function and acute kidney injury (AKI).[15] It has been shown that severe immunosuppression and high viral loads are the most important risk factors for all forms of renal injury.[16] The most widely described renal disease in HIV infected population is HIVAN, which is characterized by nephrotic range proteinuria, collapsing glomerulopathy and microcystic tubular dilatation. HIVAN has strong racial predilection (African American) and is less well reported from other populations. Studies related to renal diseases in HIV from Thailand emphasized that HIVAN was uncommon in their cohorts.[1718] Case reports from India have described collapsing focal segmental glomerulosclerosis (FSGS) in adults with HIV but not classic HIVAN.[192021]
Centers in India have described their experience in managing large cohorts of children living with HIV, but they remain silent on any form of renal disease.[2223] Reports of established FSGS/HIVAN in pediatric population are limited.[2223242526] However, recent literature among Indian adult population has suggested that contrary to the belief, renal dysfunction in the form of proteinuria is common among HIV population up to 17.3%.[19] These studies have also shown that renal disease has a broad spectrum and is not confined to HIVAN alone.[19] Therefore, a need was felt to conduct a study to investigate early renal dysfunction in children living with HIV using monitoring parameters, which predict tubular injury like uNGAL, eGFR and microalbuminuria for glomerular injury.
Shah et al. in 2012 had reported eGFR abnormality to be the most common renal manifestation in their cohort of children with HIV infection.[27] They reported abnormal eGFR (90.6 + 22.1ml/min, range 52.9ml/min-156.2ml/min) in 44% of HIV infected ART naïve children. In our study, 14 children (23.3%) had low eGFR using the Schwartz formula. Mean eGFR in the severely immunosuppressed group was significantly lower than other groups (P value = 0.028) [Table 1]. A strong association of HIV viral load and decline in kidney function has been documented.[28] HIV directly infects all cell types in the kidney and actively replicates there, resulting in progressive decline in renal function.[29303132] The low eGFR in this sub-group is likely a reflection of underlying high viremic state, though viral loads were not available to corroborate.
Microalbuminuria is an established early marker of renal damage in HIV infected population. Worldwide, studies on microalbuminuria in children with HIV infection have shown a prevalence ranging from 12 to 30%.[457] It was noted in ART naïve children with severe immunosuppression warranting initiation of ART. Most of these studies are from USA and Africa, and highest prevalence of microalbuminuria has been reported in patients belonging to African American race.[47] Similarly, a single study among children with HIV infection by Gupta et al. has shown proteinuria to be present in 11.5% and microalbuminuria in 10.6% of their cohort.[33] Prevalence of microalbuminuria noted in our study was only 3.3%, which was lower when compared to the other studies. There are considerable differences in patient population between our study and that by Gupta et al. Ours was a smaller cohort (60 vs. 183) and included children with relatively well-preserved kidney function. The cohort of children in the previous study included 82% children on ART while 66% children in our study were ART naïve. Drug related toxicity, immune reconstitution, host immune status as well as ethnic differences may be responsible for these differences.
Microalbuminuria has been associated with low CD4 counts and low CD4 percentages in children in previous studies done in USA and African continent.[47] Similar association has been observed in studies from India as well.[3435] In our study, even though a statistically significant correlation with absolute CD4+ T cell counts or CD4 percentages could not be established with ACR values, a higher mean ACR values were found in the severely immunosuppressed children. This is likely because of severe immune suppression with a high viral load leading to early renal dysfunction in this group. Virus-specific mechanisms, host immune status, and mechanisms of the host response (such as inflammation) may influence the development of kidney disease.[363738]
Microalbuminuria has been demonstrated to be an independent risk factor for mortality in adult HIV infected women.[6] In HIV infected children, such direct correlation with mortality has not been established with microalbuminuria or proteinuria. Similarly, uNGAL and ACR have also been associated with higher mortality in HIV infected women.[39] It is interesting to note that in our study cohort, two children expired at the end of study period. Both these children had high uNGAL expression (above 1,000 mcg/g).
Recent reports have suggested utility of uNGAL in detection of early tubular injury. It has been postulated to be an effective non-invasive marker for HIVAN where facilities for renal biopsy are not easily available.[10] Studies in HIV infected adults have shown that high uNGAL levels correlate with HIVAN when compared to non-HIVAN renal disease.[10] The other observation reported is that of high uNGAL values in HIV infected children with proteinuria (both gross and trace) when compared to asymptomatic HIV positive controls (exact values not given).[40]
We have found a statistically significant higher uNGAL values (P value 0.003) in our study population when compared to the normal population data [Table 2]. The HIV infected children expressed 1.5 times higher uNGAL when compared to the normal population. This result of higher uNGAL expression in HIV infected children further reiterates the fact that tubular dysfunction does occur in children with HIV infection and requires close monitoring for early identification of kidney disease. Furthermore, it is important to note that eGFR may remain normal during early stage of tubular injury. Therefore, uNGAL is a valuable tool that predict tubular injury.
Studies on uNGAL and HIVAN in children are scarce. Among adults, it has been shown that uNGAL levels significantly correlate with viral loads and has been suggested that uNGAL expression is decreased by HAART.[9] Paragas et al. had also demonstrated that patients with HIVAN had low CD4 counts, high serum creatinine compared to controls.[9]
The mean uNAGL and mean uNGAL/creatinine ratio was significantly higher in ART naïve children with severe immunosuppression in our study [Table 2]. This association was further reiterated with the finding that uNGAL expression had significant negative correlation with absolute CD4+T cell counts and CD4 percentages (correlation co-efficient of -0.0.310 and -0.532 respectively). The tubular injury in HIV infection is related to cell cycle dysregulation, increase cytokines expression and elevation of profibrotic mediators leading to tubulointerstitial inflammation, tubular cell injury and death.[41]
uNGAL is an excellent biomarker that predicts early tubular injury and higher level indicates progression of chronic kidney disease.[42] Increased uNGAL also observed during concomitant inflammation or sepsis. Therefore, it is prudent to exclude all these clinical conditions before uNGAL measurement in children with HIV infection. The routine use of uNGAL is still difficult to recommend because of its higher cost in comparison to other renal biomarkers.
In our study cohort, persistent microalbuminuria and higher uNGAL was seen in children with severe immunosuppression warranting initiation of ART. This is likely because of high viral load causing renal injury in this group in comparison to children with stable CD4 counts and children on ART for more than 1 year.
Viral load estimation could not be carried in our patients because of resource constraints. This along with small sample size and short follow-up duration are major limitations of this study. Furthermore, we followed a convenient sampling technique to recruit children, therefore our cohort is not widely representative. In addition, uNGAL was measured only at baseline and urine microalbumin was not measured more than once at each instance, so transient microalbuminuria could not be ruled out.
Conclusion
To conclude, this pilot study gives an insight into the prevalence of renal dysfunction in pediatric HIV population. It shows that renal damage does occur early in the course of the disease and it may not be clinically overt.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge the NACO (National AIDS Control Organization) for providing ART free of cost to the patients.
References
- Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667-78.
- [Google Scholar]
- HIV-associated nephropathy: A diagnosis in evolution. Nephrol Dial Transplant. 2013;27:3969-72.
- [Google Scholar]
- Microalbuminuria predicts overt proteinuria among patients with HIV infection. HIV Med. 2010;11:419-26.
- [Google Scholar]
- Microalbuminuria in children with human immunodeficiency virus (HIV) infection in Port Harcourt, Nigeria. Niger J Med. 2010;19:298-301.
- [Google Scholar]
- Microalbuminuria is associated with all-cause and AIDS mortality in women with HIV infection. J Acquir Immune Defic Syndr. 2010;55:73-7.
- [Google Scholar]
- Renal manifestations and associated factors among HIV infected children at Muhimbili National Hospital, Dar es Salaam, Tanzania. BMC Infect Dis. 2012;12(Suppl 1):O11.
- [Google Scholar]
- Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012-24.
- [Google Scholar]
- Urinary NGAL marks cystic disease in HIV-associated nephropathy. J Am Soc Nephrol. 2009;20:1687-92.
- [Google Scholar]
- Urinary NGAL is a useful clinical biomarker of HIV-associated nephropathy. Nephrol Dial Transplant. 2011;26:2387-90.
- [Google Scholar]
- 2011 Global IDF/ISPAD Guideline for Diabetes in Childhood and Adolescence-International Society for Pediatric and Adolescent Diabetes. Available from: https://www.ispad.org/page/idfispadguidenews/2011-Global-IDFISPAD-Guideline-for-Diabetes-in-Childhood-and-Adolescen.html.
- [Google Scholar]
- A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259-63.
- [Google Scholar]
- National kidney foundation's kidney disease outcomes quality initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics. 2003;111:1416-21.
- [Google Scholar]
- The types of renal disease in the acquired immunodeficiency syndrome. N Engl J Med. 1987;316:1062-8.
- [Google Scholar]
- Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clin J Am Soc Nephrol. 2013;8:1524-32.
- [Google Scholar]
- Pattern of glomerular involvement in human immunodeficiency virus-infected patients: An Italian study. Am J Kidney Dis. 1995;26:446-53.
- [Google Scholar]
- Spectrum of renal lesions in HIV patients. J Assoc Physicians India. 2000;48:1151-4.
- [Google Scholar]
- Collapsing glomerulopathy in an HIV-positive patient in a low-incidence belt. India J Nephrol. 2010;20:211-3.
- [Google Scholar]
- HIV associated renal disease: A pilot study from north India. Indian J Med Res. 2013;137:950-6.
- [Google Scholar]
- Clinical profile of 516 children affected by HIV in a tertiary care centre in northern India: 14 years of experience. Trans R Soc Trop Med Hyg. 2009;103:627-33.
- [Google Scholar]
- HIV-associated nephropathy and end-stage renal disease in children in the United States. Pediatr Nephrol. 2004;19:808-11.
- [Google Scholar]
- HIV-associated nephropathy in the setting of maximal virologic suppression. Pediatr Nephrol. 2011;26:973-7.
- [Google Scholar]
- Human immunodeficiency virus-associated nephropathy (HIVAN) in Nigerian children. Pediatr Nephrol. 2008;23:117-22.
- [Google Scholar]
- Renal manifestations of HIV infected highly active antiretroviral therapy naive children in India. World J Pediatr. 2012;8:252-5.
- [Google Scholar]
- Recent progress in HIV-associated nephropathy. J Am Soc Nephrol. 2002;13:2997-3004.
- [Google Scholar]
- Nephropathy in humanimmunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest. 1997;100:84-92.
- [Google Scholar]
- Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344:1979-84.
- [Google Scholar]
- Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11:2079-87.
- [Google Scholar]
- Prevalence of Asymptomatic Microalbuminuria in HIV Positive Children in India. Indian J Pediatr. 2017;84:417-9.
- [Google Scholar]
- Correlation of CD4 counts with renal disease in HIV positive patients. Saudi J Kidney Dis Transpl. 2008;19:603-7.
- [Google Scholar]
- Molecular mechanisms of injury in HIV-associated nephropathy. Available from http://wwwfrontiresinorg/articles/103389/fmed 201800177/full
- [Google Scholar]
- Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203.
- [Google Scholar]
- HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172.
- [Google Scholar]
- Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women's Interagency HIV Study (WIHS) HIV Med. 2014;15:291-300.
- [Google Scholar]
- Iron-related proteins: Candidate urinebiomarkers in childhood HIV-associated renal diseases. Clin J Am Soc Nephrol. 2009;4:763-71.
- [Google Scholar]
- HIV- associated nephropathy: Pathogenesis. Curr Opin Nephrol Hypertens. 2011;20:306-11.
- [Google Scholar]
- Emerging biomarkers of chronic kidney disease in children. Pediatr Nephrol. 2018;33:925-33.
- [Google Scholar]







