Translate this page into:
Association of VEGF -2549 I/D and VEGF +936 C/T Polymorphisms with Chronic Kidney Disease in North-West Indian Patients
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Chronic kidney disease (CKD) is a complex multifactorial disease in which both genetic and environmental factors influence the onset, development and progression of disease. The genetic variations in the vascular endothelial growth factor (VEGF) can influence levels of VEGF protein expression, and thus, susceptibility to progression of kidney diseases. The aim of the present study was to evaluate the association of VEGF-2549 I/D and VEGF +936 C/T polymorphisms in CKD stage V patients from North-West India.
Methods:
In this case-control study, 166 patients and 166 controls were analyzed. DNA samples were screened for VEGF -2549I/D and VEGF +936 C/T polymorphisms using polymerase chain reaction–based (PCR) methods.
Results:
The genotype frequency of VEGF -2549 I/D was significantly different between patients and controls (P < 0.05). ID genotype of VEGF -2549 I/D polymorphism was significantly associated with decreased risk of CKD (P = 0.009). Genetic model analysis of VEGF -2549 I/D polymorphism revealed a significantly decreased risk of CKD in co-dominant (P = 0.009), dominant (P = 0.021), and over-dominant (P = 0.012) models. Genotype and allele frequency of VEGF +936 C/T polymorphism was not significantly different between the patient and control groups. Genotype combination analysis revealed that ID-CT genotype combination of VEGF -2549 I/D and VEGF +936 C/T polymorphisms was associated with decreased CKD risk (P = 0.047).
Conclusion:
VEGF -2549 ID genotype and ID-CT genotype combination of VEGF -2549 I/D and VEGF +936 C/T polymorphisms was significantly associated with reduced CKD risk in North-West Indians.
Keywords
Kidney disease
polymorphism
VEGF
Introduction
Chronic kidney disease (CKD) is a complex multifactorial disease in which both genetic and environmental factors influence the onset, development, and progression of disease. The progressive and irreversible loss of renal function results in end-stage renal disease (ESRD), necessitating renal replacement therapies (RRTs) for life-sustenance. With a global burden of 13.7%, it is a major risk factor for cardiovascular morbidity and mortality worldwide.[1] Etiological basis of the disease is associated with various pathogenic factors, namely, glomerulonephritis, diabetes, hypertension, and urologic disorders. However, progressive renal microvascular dysfunction, which initiates and promotes interstitial fibrosis, tubular atrophy, and glomerulosclerosis, is a universal pathologic feature of CKD.[2] The microvasculatures of glomerular and peritubular capillaries are critical in kidney disease. Damage to glomerular capillary vasculature leads to proteinuria, and to the peritubular capillary results in chronic hypoxia followed by tubulointerstitium fibrosis.[34] Vascular endothelial growth factor (VEGF), a major regulator of blood vessel growth plays a pivotal role in promoting endothelial survival, functional and morphological maintenance of these microvascular networks.[5] In kidneys, VEGF is essential for growth and proliferation of glomerular and peritubular endothelial cells, and thus, maintenance of fenestrae in endothelial cells of glomerular capillaries.[6]
Human VEGF or VEGFA is located on 6p12 spans 16,272 bp and consists of eight exons.[7] It is reported to be highly polymorphic in the promoter region, 5′ untranslated region (UTR), and 3′ UTR.[8] There are reports on the association of these genetic variations with altered serum and urine VEGF levels[89] and diseases including diabetic nephropathy,[1011] glomerulonephritis,[12] ESRD,[13]and acute renal allograft rejection.[141516] VEGF-2549 insertion/deletion and VEGF +936 C/T have been implicated in a number of diseases with angiogenic basis, and hence are polymorphisms of particular interest.[171819]
The genetic variations in the VEGF can influence levels of VEGF protein expression, and thus susceptibility to progression of kidney diseases. The global burden of CKD is increasing and availability of basic life-sustaining RRTs is limited due to economic constraints.[20] Moreover, screening of VEGF polymorphisms can help identify at-risk individuals for graft rejection prior to transplantation.[1416] Literature regarding the role of VEGF -2549 I/D and VEGF +936 C/T polymorphisms in various diseases is vast, but is limited to renal complications, especially CKD stage V patients with different etiologies and hemodialysis durations. Therefore, the present study was an attempt to evaluate the association of VEGF-2549 I/D and VEGF +936 C/T polymorphisms in CKD stage V patients from North-West India.
Methodology
Selection of subjects and collection of genetic material
The present case-control study was carried out after receiving approval from the Institutional Ethics Committee. The physician identified 166 unrelated adult CKD stage V patients (112 males and 54 females) on or starting hemodialysis therapy who were contacted from local hospitals of Amritsar, Punjab. Related, minors, and individuals who were seropositive for hepatitis C or B or HIV or had bacterial infections or any cancer, were not included in this study. Unrelated, healthy, age and gender-matched 166 individuals (107 males and 59 females) from the general population belonging to same geographical area formed the control group. The eGFR values based on the creatinine levels were used to establish the healthy kidney status of the controls. The demographic characteristics, clinical profile and disease history of all the subjects were recorded on a pre-designed structured proforma. After informed written consent, 5 ml venous blood was collected from each subject in EDTA vial.
Analysis of VEGF-2549 I/D and VEGF +936 C/T polymorphisms
Genomic DNA was extracted from the peripheral blood using standard phenol chloroform method[21] with few modifications. The DNA samples were quantified on 1% agarose gel and screened for VEGF-2549I/D and VEGF +936 C/T polymorphisms. The VEGF-2549I/D promoter polymorphism was screened by direct PCR whereas 3'UTR polymorphism VEGF +936 C/T was screened usingpolymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The region of VEGF promoter harboring -2549I/D polymorphism was amplified using Forward: 5′-GCTGAGGATGGGGCTGACTAGGTA-3′ and Reverse: 5′GTTTCTGACCTGGC TATTTCCAGG-3′primers. The PCR reaction with a total volume of 10μl, contained 50ng of genomic DNA, 1X Taq buffer with 1.5mM MgCl2 (Merck, India), 4 picomoles of each primer (Sigma), 0.4μl of dNTPs mix (Merck, India), and one unit of Taq polymerase (Merck, India). For VEGF -2549I/D analysis, PCR conditions were the following: initial denaturation at 95°C for 5 minutes followed by 35 cycles with denaturation at 95°C for 45 seconds, annealing at 55°C for 30 seconds, extension at 72°C for 45 seconds, and final extension at 72°C for 10 minutes. The amplified products were analyzed on 2.4% ethidium bromide-stained agarose gel. A band of 229 bp represents I allele whereas band of 211bp represents D allele[Figure 1].
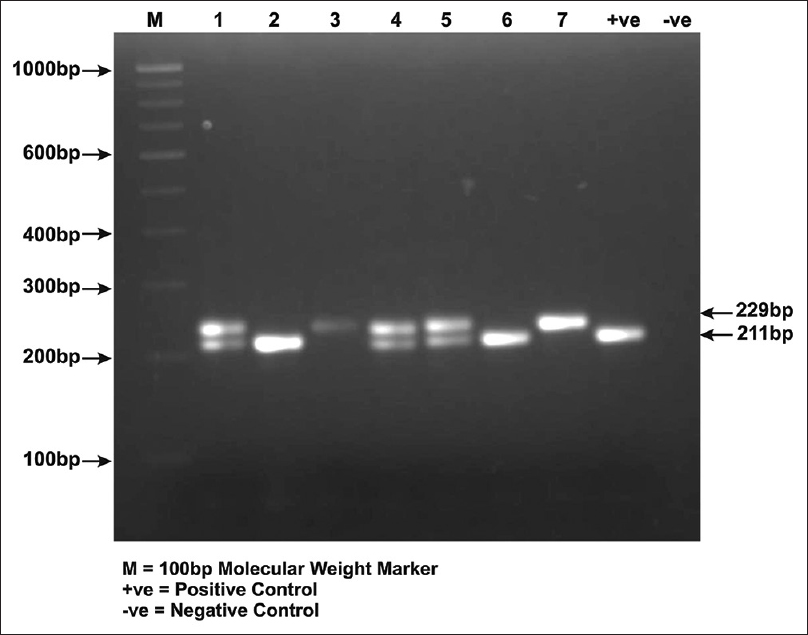
- A photograph of 2.4% ethidium bromide –stained agarose gel demonstrating VEGF -2549I/D polymorphism. Lane 1, 4, 5 represents ID genotype, Lane 2, 6, +ve control represents DD genotype and Lane 3, 7 represents II genotype
The specific region of VEGF containing +936 C/T polymorphism was amplified using Forward: 5′-AGGAAGAGGAGACTCTGCGCAGAGC-3′ and Reverse: 5′-TAAATGTATGTATGTGGG TGGGTGTGTCTACAGG-3′ primers. PCR reaction with a total volume of 15μl, contained 50ng of genomic DNA, 1X Taq buffer, 1mM MgCl2 (Merck, India), 6 picomoles of each primer (Sigma), 0.2μl dNTPs mix (Merck, India) and one unit of Taq polymerase (Merck, India). The amplification conditions used were: initial denaturation at 95°C for 5 minutes, followed with a denaturation at 95°C for 45 seconds, annealing at 59°C for 30 seconds and an extension at 72°C for 45 seconds for 35 cycles with final extension at 72°C for 10 minutes. The amplified PCR products of 217bp were digested with NlaIII restriction enzyme (New England Biolabs, Beverly, MA) at 37°C overnight. Restriction digestion reaction products were analyzed on 2.4% ethidium bromide–stained agarose gel. Two fragments of 122bp and 85bp indicates +936T allele, whereas the undigested fragment of 207bp represents +936C allele [Figure 2]. The details of reaction composition and conditions used have been presented in our published study.[22] To ensure genotyping accuracy, positive and negative controls were used in every batch of reaction. The PCR assay-based results were validated by reanalyzing the 10% of randomly selected samples.

- A photograph of 2.4% ethidium bromide–stained agarose gel demonstrating restriction digestion pattern of VEGF +936C/T polymorphism. Lane 1, 3, 5 represents CC genotype, Lane 2, 4 represents CT genotype and +ve control represents TT genotype
Statistical analyses
The data on variables are expressed as number or as percentage, and as mean ± standard deviation. Differences between the patient and control groups were analyzed by independent Student's t test while multiple comparisons were carried out using one-way ANOVA. The allele frequencies were tested for the Hardy–Weinberg equilibrium (HWE) for both patients and controls using the Chi-square test. This test was also used to evaluate the differences in the VEGF genotype and allele frequencies between the patient and control groups, and also as a function of various general, demographic, and clinical parameters. Odds ratio (OR) and its 95% confidence interval (CI) were used to assess the association between genotypes and alleles with the disease risk. Haplotypes were constructed using SNPStats.[23] Statistical significance level was set at P < 0.05.
Results
General demographic and clinical profile of subjects
The patient group (n = 166) comprised of unrelated patients in CKD stage V in the age range of 18–80 years (mean = 50.87 ± 13.36 years) with high frequency of males (67.47%). Mean age of unrelated healthy individuals was 48.24 ± 12.23 years [Table 1]. About 74.69% of the patients were on hemodialysis therapy for 8 months to 6 years. Ongoing hemodialysis therapy comprised of groups on once-a-week (23.49%), twice-a-week (25.30%), thrice-a-week (12.05%), and fortnightly (15.66%) regimens with 25.30% yet to initiate hemodialysis. Serum creatinine (7.37 ± 3.37 mg/dl) and urea (125.46 ± 34.90 mg/dl) levels were elevated in the patients. Patients were on prescribed medications from past 9.24 ± 0.70 years.
| Characteristics | Categories/Types | Patients n (%) | Controls n (%) |
|---|---|---|---|
| Age (years) | ≤50 | 80 (48.19) | 94 (56.63) |
| >50 | 86 (51.80) | 72 (43.37) | |
| Mean | 50.87±13.36 | 48.24±12.24 | |
| Gender | Male | 112 (67.47) | 107 (64.46) |
| Female | 54 (32.53) | 59 (35.54) | |
| Socioeconomic status[24] | Upper | 10 (6.02) | 04 (2.41) |
| Upper Middle | 52 (31.33) | 52 (31.33) | |
| Lower Middle | 46 (27.71) | 54 (32.53) | |
| Upper Lower | 58 (34.94) | 56 (33.73) | |
| Diet | Vegetarian | 119 (71.69) | 126 (75.90) |
| Non-vegetarian | 47 (28.31) | 40 (24.10) | |
| Smoking habit | Yes | 05 (3.01) | 07 (4.22) |
| No | 161 (96.99) | 159 (95.78) | |
| Alcohol consumption | Yes | 12 (7.23) | 35 (21.08) |
| No | 118 (71.08) | 125 (75.30) | |
| Ex-drinker | 36 (21.67) | 06 (3.61) | |
| Mobile usage | Yes | 129 (77.71) | 137 (82.53) |
| No | 37 (22.29) | 29 (17.47) | |
| General obesity[25] | Underweight | 13 (7.83) | 04 (2.41) |
| Normal | 95 (57.23) | 76 (45.78) | |
| BMI (kg/m²) | Overweight | 30 (18.07) | 26 (15.66) |
| Obese | 28 (16.87) | 60 (36.14) | |
| Mean | 22.09±3.20 | 24.28±4.12 | |
| Blood Pressure | SBP (mmHg) | 137.49±16.77 | 131.59±14.14 |
| DBP (mmHg) | 85.72±8.05 | 84.78±10.87 | |
| PP | 51.77±12.59 | 46.81±10.33 | |
| MAP | 102.98±10.09 | 100.39±11.03 | |
| Creatinine levelsǂ (mg/dl) | 7.37±3.37 | 1.12±0.61 | |
| Urea levels (mg/dl)# | 125.46±31.78 | - | |
| Comorbidity | Diabetes | 51 (30.72) | - |
| Hypertension | 37 (22.29) | - | |
| Diabetes + Hypertension | 13 (7.83) | - | |
| Unknown | 65 (39.16) | - | |
| Dialysis | ≤1 | 57 (34.34) | - |
| Duration | >1 | 67 (40.36) | - |
| (Years) | Non-Dialyzed | 42 (25.30) | - |
BMI- Body Mass Index; CKD-EPI- Chronic kidney disease epidemiology equation; DBP- Diastolic Blood Pressure; eGFR- estimated glomerular filtration rate; MAP- Mean Arterial Pressure; SBP-Systolic Blood Pressure; PP- Pulse Pressure. ǂNormal range 0.80-1.40 mg/dl for males and 0.60-1.40 mg/dl for females. #Normal range 8-20 mg/dl; Data not available for controls. $Calculated using variables of age, gender, and serum creatinine levels (http://www.nkdep.nih.gov/professionals/gfr_calculators/index.htm)
VEGF-2549 I/D and VEGF +936 C/T polymorphisms and disease risk
Genotype and allele frequencies of VEGF-2549 I/D and VEGF +936 C/T polymorphisms in patient and control groups are detailed in Table 2. The genotype distributions of VEGF -2549 I/D and VEGF +936 C/T polymorphisms were in HWE (P > 0.05; except VEGF -2549 I/D in controls). The frequency of DD (33.73 vs 22.29%), ID (46.99 vs 60.84%) and II (19.28 vs 16.87%) genotypes of VEGF -2549 I/D was significantly different between patients and controls (P < 0.05). ID genotype of VEGF -2549 I/D polymorphism was significantly associated with decreased risk of CKD (P = 0.009). The frequency of CC (82.53% vs 83.13%) and CT (16.27% vs 16.87%) genotypes of VEGF +936 C/T polymorphism was not significantly different between the patient and control groups. The variant genotype TT was only observed in the patient group (1.20%). The frequency of T allele was slightly higher in patients (9.34%) compared to controls (8.43%).
| Variant | Patients n (%) | Controls n (%) | χ2 (P) | OR (95%CI) | P |
|---|---|---|---|---|---|
| -2549 I/D (rs35569394) | |||||
| Genotype | |||||
| DD | 56 (33.73) | 37 (22.29) | 7.104 (0.029) | Reference | |
| ID | 78 (46.99) | 101 (60.84) | 0.510 (0.307-0.849) | 0.009 | |
| II | 32 (19.28) | 28 (16.87) | 0.775 (0.392-1.454) | 0.401 | |
| Allele | |||||
| D | 190 (57.23) | 175 (52.71) | 1.193 (0.275) | Reference | |
| I | 142 (42.77) | 157 (47.29) | 0.833 (0.613-1.131) | 0.242 | |
| Hardy-Weinberg Equilibrium | χ2=0.268 | χ2=8.068 | |||
| P=0.605 | P=0.005 | ||||
| +936 C/T (rs3025039) | |||||
| Genotype | |||||
| C/C | 137 (82.53) | 138 (83.13) | 2.022 (0.364) | Reference | |
| C/T | 27 (16.27) | 28 (16.87) | 0.971 (0.544-1.733) | 0.926 | |
| T/T | 02 (1.20) | - | - | ||
| Allele | |||||
| C | 301 (90.66) | 304 (91.57) | 0.074 (0.785) | Reference | |
| T | 31 (9.34) | 28 (8.43) | 1.118 (0.655-1.909) | 0.683 | |
| Hardy-Weinberg Equilibrium | χ2=0.257 | χ2=1.408 | |||
| P=0.612 | P=0.235 | ||||
Values in bold are significant; OR: Odds Ratio, CI: Confidence Interval
The genetic model analysis of VEGF -2549 I/D polymorphism revealed a significant decreased risk of CKD in co-dominant (OR: 0.510, 95% CI: 0.307–0.849; P = 0.009), dominant (OR: 0.563, 95% CI: 0.346-0.917; P = 0.021) and over-dominant (OR: 0.570, 95% CI: 0.369–0.882; P = 0.012) models [Table 3]. However, none of the genetic models of VEGF +936 C/T polymorphism showed any significant disease association.
| Variant | Model | Genotypes | Patients n (%) | Controls n (%) | OR (95% CI) | P |
|---|---|---|---|---|---|---|
| -2549 I/D (rs35569394) | Co dominant | DD | 56 (33.73) | 37 (22.29) | Reference | |
| ID | 78 (46.99) | 101 (60.84) | 0.510 (0.307-0.849) | 0.009 | ||
| II | 32 (19.28) | 28 (16.87) | 0.775 (0.392-1.454) | 0.401 | ||
| Dominant | DD | 56 (33.73) | 37 (22.29) | Reference | ||
| ID + II | 110 (66.27) | 129 (77.71) | 0.563 (0.346-0.917) | 0.021 | ||
| Recessive | DD+ID | 134 (80.72) | 138 (83.13) | Reference | ||
| II | 32 (19.28) | 28 (16.87) | 1.177 (0.672-2.061) | 0.569 | ||
| Over dominant | DD + II | 88 (53.01) | 65 (39.16) | Reference | ||
| ID | 78 (46.99) | 101 (60.84) | 0.570 (0.369-0.882) | 0.012 | ||
| +936 C/T (rs3025039) | Co dominant | CC | 137 (82.53) | 138 (83.13) | Reference | |
| CT | 27 (16.27) | 28 (16.87) | 0.971 (0.544-1.733) | 0.926 | ||
| TT | 02 (1.20) | - | ||||
| Dominant | CC | 137 (82.53) | 138 (83.13) | Reference | ||
| CT + TT | 29 (17.47) | 28 (16.87) | 1.043 (0.589-1.846) | 0.884 | ||
| Recessive | CC + CT | 164 (98.80) | 166 (100) | Reference | ||
| TT | 02 (1.20) | - | NC | |||
| Over dominant | CC + TT | 139 (83.73) | 138 (83.13) | Reference | ||
| CT | 29 (17.47) | 28 (16.87) | 1.028 (0.581-1.819) | 0.924 | ||
Values in bold are significant; OR: Odds Ratio, CI: Confidence Interval; NC: Not calculated
The frequency of distribution of VEGF -2549 I/D and VEGF +936 C/T polymorphisms as a function of general demographic (age, gender) and disease-specific variables showed no association with the disease (data not shown). Genotype combination analysis [Table 4] showed that ID genotype of VEGF -2549 I/D and CT genotype of VEGF +936 C/T polymorphisms was associated with decreased CKD risk (OR: 0.450, P = 0.047). Haplotypes constructed for VEGF-2549 I/D and VEGF +936 C/T polymorphisms did not reveal any significant disease-risk association [Table 4]. Analysis of association of VEGF -2549 I/D and VEGF +936 C/T polymorphism with creatinine level showed non-significant results (data not shown).
| Patient n(%) | Control n(%) | OR (95% CI) | P | |
|---|---|---|---|---|
| Genotype Combinations# | ||||
| DD-CC | 50 (30.12) | 37 (22.29) | Reference | |
| ID-CC | 63 (37.95) | 78 (46.99) | 0.598 (0.349-1.025) | 0.061 |
| II-CC | 24 (14.46) | 23 (13.86) | 0.772 (0.379-1.575) | 0.477 |
| DD-CT | 06 (3.61) | - | NC | |
| ID-CT | 14 (8.43) | 23 (13.86) | 0.450 (0.205-0.991) | 0.048 |
| II-CT | 07 (4.22) | 05 (3.01) | 1.036 (0.305-3.523) | 0.955 |
| Haplotypes# | ||||
| D-C | 0.540 | 0.527 | Reference | |
| I-C | 0.367 | 0.389 | 0.925 (0.669-1.278) | 0.635 |
| I-T | 0.061 | 0.084 | 0.733 (0.401-1.340) | 0.313 |
| D-T | 0.032 | 0 | NC | |
Values in bold are significant; #VEGF -2549 I/D and VEGF +936 C/T; OR: Odds Ratio, CI: Confidence Interval. NC: Not calculated
Discussion
VEGF is essential for maintenance of glomerular filtration barrier and its dysregulation has been reported to be associated with various glomerular and associated diseases.[26] In the present case-control study, VEGF -2549 I/D promoter and VEGF +936 C/T 3′UTR polymorphism were screened in 166 CKD patients and 166 controls. We observed that ID genotype of VEGF -2549 I/D polymorphism was significantly associated with decreased risk of CKD. In the literature, it has been reported that VEGF promoter and 3′UTR polymorphism are associated with varying VEGF production. It has also been documented that D allele of VEGF -2549 I/D polymorphism was associated with 1.95-fold increased transcriptional activity as compared to I allele.[27] DD genotype and D allele of VEGF -2549 I/D polymorphism were associated with susceptibility to diabetic nephropathy in British Caucasoid patients.[25] DD genotype and D allele was associated with increased risk of hypertensive nephrosclerosis in North Indians.[13] Significant association of D allele with increased risk to diabetic nephropathy has been reported in North Indian population.[11]
A comparison of genotype frequencies of the present study with those reported in literature in kidney disease revealed that genotype frequencies of VEGF -2549 I/D polymorphism observed in the present study are similar as reported in ESRD patients from North India.[13]The genotype frequencies of VEGF +936 C/T polymorphism observed in the presented study were comparable with Caucasian kidney graft patients.[14] ID-CT genotype combination of VEGF -2549 I/D and VEGF +936 C/T polymorphisms was associated with decreased CKD risk in the present study. It has been reported that carriers of the T allele of VEGF +936 C/T polymorphism have significantly lower VEGF plasma levels as compared to non-carriers.[9] CT and TT genotype and T allele of VEGF +936 C/T polymorphism was associated with increased risk of kidney disease patients with different etiologies like chronic glomerulonephritis, chronic interstitial nephritis, hypertensive nephrosclerosis in North Indians.[13]
As a function of disease-etiologies (diabetic nephropathy, hypertensive nephropathy, miscellaneous), non-significant differences in frequency distribution of the studied polymorphisms were observed in the present study. Similar to our findings, no association of VEGF +936 C/T polymorphism with glomerulonephritis was observed in Turkish population.[12] Stratification of the patients on the basis of demographic and clinical variables revealed non-significant differences. Though there was a preponderance of male patients in the present study, non-significant association of VEGF -2549 I/D and VEGF +936 C/T polymorphisms was observed in males. In Japanese patients it has been demonstrated that CC genotype of VEGF +936 C/T polymorphism was not only associated with risk to ESRD but also associated with increased VEGF levels and mRNA stability in males.[28]The VEGF +936 CT genotype and T allele have been reported to be associated with good outcome in renal transplantation.[14] T allele was also found to be associated with acute renal allograft rejection.[29] Significant association of VEGF-2549 DD genotype with graft failure and protective association of VEGF +936 CC genotype in kidney allograft recipients was reported in North Indians.[16] The intrarenal VEGF therapy at both, preventive and interventional stages has been reported to be associated with improved renal microvasculature and function and reduction in renal fibrosis.[30] Therefore, screening of the VEGF polymorphisms holds relevance as it is an important angiogenic factor implicated in renal pathologies.
Conclusion
Present case-control study revealed that VEGF -2549 ID genotype and ID-CT genotype combination of VEGF -2549 I/D and VEGF +936 C/T polymorphisms was significantly associated with decreased CKD risk in North-West Indians.
Financial support and sponsorship
This study is partially supported by the MHRD grant under RUSA scheme sanctioned to Kamlesh Guleria and Vasudha Sambyal. Postdoctoral fellowship to Gurleen Kaur Tung under RUSA scheme is dully acknowledged. The clinical classification of patients by late Dr. PS Mokha, Mokha Hospital and Kidney Care Centre Amritsar is gratefully acknowledged.
Conflicts of interest
There are no conflicts of interest.
References
- Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3-15.
- [Google Scholar]
- Origin of myofibroblasts and cellular events triggering fibrosis. Kid Int. 2015;87:297-307.
- [Google Scholar]
- Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17-25.
- [Google Scholar]
- Etiopathology of chronic tubular, glomerular and renovascular nephropathies: Clinical implications. J Transl Med. 2011;9:1-26.
- [Google Scholar]
- Vascular endothelial growth factor: A new player in the pathogenesis of renal fibrosis. Curr Opin Nephrol Hypertens. 2003;12:43-9.
- [Google Scholar]
- Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707-16.
- [Google Scholar]
- VEGF-A splicing: The key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880-7.
- [Google Scholar]
- Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: Correlation with variation in VEGF protein production. Cytokine. 2000;12:1232-5.
- [Google Scholar]
- A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res. 2000;37:443-8.
- [Google Scholar]
- What is the contribution of two genetic variants regulating VEGF levels to type 2 diabetes risk and to microvascular complications? PLoS One. 2013;8:e55921.
- [Google Scholar]
- Association of 18bp insertion/deletion polymorphism, at -2549 position of VEGF gene, with diabetic nephropathy in type 2 diabetes mellitus patients of North Indian population. J Diabetes MetabDisord. 2015;14:19.
- [Google Scholar]
- The association between therapeutic outcomes and VEGF G-1154A and C-936T gene polymorphisms in patients with glomerulonephritis. Ren Fail. 2014;36:904-7.
- [Google Scholar]
- Vascular endothelial growth factor gene polymorphisms in North Indian patients with end stage renal disease. Cytokine. 2012;58:261-6.
- [Google Scholar]
- VEGF 936 C/Tgene polymorphism in renal transplant recipients: Association of the T allele with good graft outcome. HumImmunol. 2007;68:599-602.
- [Google Scholar]
- Association of HLA-G promoter and 14-bp insertion–deletion variants with acute allograft rejection and end-stage renal disease. Tissue Antigens. 2013;82:317-26.
- [Google Scholar]
- Vascular endothelial growth factor gene polymorphism is associated with long-term kidney allograft outcomes. Kidney IntRep. 2018;3:321-7.
- [Google Scholar]
- Lack of association between three vascular endothelial growth factor gene polymorphisms and systemic sclerosis: Results from a multicenter EUSTAR study of European Caucasian patients. Ann Rheum Dis. 2007;66:257-9.
- [Google Scholar]
- Vascular endothelial growth factor gene polymorphisms in North Indian patients with recurrent miscarriages. Reprod Biomed Online. 2011;22:59-64.
- [Google Scholar]
- VEGF-directed blood vessel patterning: From cells to organism. Cold Spring HarbPerspect Med. 2012;2:a006452.
- [Google Scholar]
- Molecular Cloning-A Laboratory Manual. (2nd). New York: Cold Spring Harbor Laboratory Press; 1989.
- [Google Scholar]
- Association of VEGF and VEGFR1 polymorphisms with breast cancer risk in North Indians. Tumour Biol. 2015;36:4223-34.
- [Google Scholar]
- SNPStats: A web tool for the analysis of association studies. Bioinformatics. 2006;22:1928-9.
- [Google Scholar]
- Updating income ranges for Kuppuswamy's socio-economic status scale for the year 2014. Indian J Public Health. 2015;59:156-7.
- [Google Scholar]
- Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163-9.
- [Google Scholar]
- Stability and species specificity of renal VEGF-A splicing patterns in kidney disease. PLoS One. 2016;11:e0162166.
- [Google Scholar]
- Polymorphisms of the vascular endothelial growth factor and susceptibility to diabetic microvascular complications in patients with type 1 diabetes mellitus. J Diabetes Complications. 2003;17:1-6.
- [Google Scholar]
- Functional polymorphisms in the vascular endothelial growth factor gene are associated with development of end-stage renal disease in males. J Am Soc Nephrol. 2006;17:823-30.
- [Google Scholar]
- Impact of vascular endothelial growth factor single nucleotide polymorphism association on acute renal allograft rejectionNephron. 2015;129:91-6.
- Vascular endothelial growth factor therapy for the kidney: Are we there yet? J Am Soc Nephrol. 2016;27:1-3.
- [Google Scholar]







