Translate this page into:
Clinical Profile, Histopathology, and Outcomes in Infection-Related Glomerulonephritis – Single-Center Experience
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Infection-related glomerulonephritis (IRGN) is an important source of renal morbidity with adverse outcomes in adults. Data from large centers in India is lacking on this common, yet poorly understood entity.
Materials and Methods:
We performed a prospective observational study of all patients diagnosed with IRGN at our center over a 3-year period between 2017 and 2019. “Typical IRGN” patients were diagnosed based on clinical and laboratory assessment; others underwent renal biopsy. Renal and patient survival outcomes were assessed in addition to factors that help predict outcomes.
Results:
One hundred and twenty-five patients with a diagnosis of IRGN were included in the study, including 86 patients who underwent renal biopsy. This represented 24% of all biopsies during this time period, and IRGN was the most common nondiabetic kidney disease identified in diabetic biopsies at our center. Female preponderance and a seasonal variation were striking. Atypical sources of infection like otomycosis, tooth abscess, and dengue virus infection were noted. Male gender and diabetes were important risk factors for severe disease. Rapidly progressive glomerulonephritis (RPGN), atypical serum complement profiles, and comorbid illnesses were common in adults. Though children had more benign disease and outcomes, life-threatening complications were also noted. C3 dominance was the most striking immunofluorescence (IF) finding and was associated with poorer outcomes. Crescentic IRGN was rare, and four cases of IgA-dominant IRGN were noted. Also, 24% of the cohort required renal replacement therapy. RPGN presentation of IRGN portended worst prognosis with end-stage renal disease (ESRD) in 31% and death in 22% of patients.
Conclusion:
IRGN is a common clinical entity in adults with the potential for adverse renal and survival outcomes. We have identified clinical and biopsy characteristics that are associated with ESRD and death.
Keywords
IgA-Dominant IRGN
infection-related glomerulonephritis
outcomes
renal biopsy
Introduction
Infection-related glomerulonephritis (IRGN) is frequently encountered in clinical nephrology practice. Data from major nephrology centers on its patterns and outcomes is lacking. In adults, this is fast emerging as a cause for significant renal morbidity and adverse sequelae. Diagnosis is often complex, combining clinical judgment with laboratory and histopathologic support.[12] We present our cohort of 125 patients diagnosed with IRGN and discuss the epidemiology, clinical profile, and short-term outcomes. We describe in detail the histopathologic findings on renal biopsy and discuss the pitfalls in diagnosis and the close differentials. We also contrast the behavior of this disease in the pediatric and adult populations.
Materials and Methods
We performed a prospective observational study in all patients diagnosed with IRGN at our center between January 2017 and December 2019. Institutional ethics committee approval was obtained and informed consent of all participants was documented.
A typical case of IRGN was defined by the following:
-
Acute nephritic syndrome presentation with evidence of preceding or concurrent infection
-
Low serum C3 with normal C4 levels, and its normalization by 8 weeks of presentation
-
Acute Kidney Injury (AKI) resolving and trending to baseline within 10 days of presentation
All such typical patients were diagnosed based only on clinical and laboratory criteria, and renal biopsy was not performed.
All patients who deviated from the above typical picture were subjected to a renal biopsy as per the unit protocol. The specific indications for biopsy in our unit included the following:
-
Any patient who presented with a rapidly progressive glomerulonephritis (RPGN)
-
Patients who did not have an identifiable focus of infection
-
Patients with normal serum complement levels or those with low C4 levels
-
Creatinine not trending to baseline by the 10th day of presentation
-
Nephrotic range proteinuria at baseline
-
Depressed complement C3 levels at 8 weeks post presentation
All renal biopsies were performed under real-time ultrasonography guidance using the Bard Max-Core® disposable biopsy instrument (Bard Biopsy Instruments, Georgia, USA) – 18 gauge. At least two cores were taken for light microscopy (LM) and immunofluorescence (IF) in all cases. Electron microscopy (EM) was done in select cases. All renal biopsies were reported by a single, experienced nephropathologist. All biopsies were subjected to standard processing and stained with hematoxylin–eosin, periodic acid-Schiff, Jones methenamine silver, and Masson trichrome. IF staining with fluorescein isothiocyanate (FITC)-conjugated polyclonal rabbit antihuman antibodies to IgG, IgM, IgA, C3, C1q, kappa and lambda light chains (Dako Corp., Carpinteria, CA, USA) was done on all specimens.
A detailed clinical examination was performed to identify any focus of infection. All patients underwent basic evaluation including urinalysis, complete hemogram, renal function test, liver function tests, stool analysis, chest X-ray, and abdominal ultrasound. Blood cultures and urine cultures were universal and supplemented with tissue, pus, and throat swab cultures in appropriate cases. All patients underwent ENT, dental, and cardiac assessment to identify occult foci of infection. Anti Strepolysin O (ASO) titer was also done. Anti-nuclear antibody and other serologies were performed as appropriate.
Specific case definitions
IgA-dominant IRGN was diagnosed when IgA was the dominant/codominant immune reactant among the immunoglobulin subclasses. C3 had to be high-intensity staining, usually higher than that of IgA.
RPGN1 was diagnosed if the peak creatinine value was more than 4 mg/dl and/or if there was a serial rapid rise in creatinine over a few days/weeks in a background consistent with glomerulonephritis.
RPGN superimposed on chronic kidney disease (CKD) was diagnosed in patients who fulfilled the above criteria and who had baseline proteinuria (>1 g) or elevated creatinine (>1.5 mg/dl). All patients of IRGN-RPGN who had biopsy findings of coexistent diabetic nephropathy of any Renal Pathology Society class or other chronic pathology were also included in this category.
Follow-up
All patients were followed up weekly twice for 3 weeks and then weekly for 8 weeks. Subsequently, monthly follow-up for 6 months was undertaken. Long-term follow-up was done every 3 months thereafter. Only patients with a minimum follow-up of at least 3 months were included in the study.
Treatment
All treatments were as per the unit protocol. This was a noninterventional study. All patients received supportive therapy with diuretics and antihypertensives dictated by clinical judgment. Patients with persistent proteinuria were treated with angiotensin receptor blockers or angiotensin converting enzyme inhibitors. Antibiotics were administered when there was active infection identified. Steroids were administered in a conservative approach only to patients with RPGN and no active infection.
Statistical analysis
All continuous variables were represented as mean with standard deviation or median, as appropriate. Student's t test was used to compare means. Fischer's test and Chi-square analysis were used for univariate analysis, as appropriate. Binomial logistic regression was employed for multivariate analysis. Two-tailed P value less than 0.05 was considered to be significant. Microsoft Excel and IBM-SPSS software were used for the analysis.
Results
One hundred and twenty-five patients with a diagnosis of IRGN were included in this study. One patient was a posttransplant IRGN in the graft kidney.
Demography and epidemiology
All patients were of Indian ancestry. The mean age at presentation was 37 years (17.6). Twenty-eight out of 125 (22%) participants were <18 years of age. Also, 6% of the cohort was ≤10 years and 10% was ≥60 years. There was a bimodal distribution of cases with respect to age, with peaks in the second and fourth to fifth decades [Figure 1]. There were more females (59%) than males in a 3:2 ratio. A distinct seasonal variation in case frequency was noted. Cases clustered during the last 4 months (September to December) of the year [Figure 2]. At baseline, 18% had diabetes, 16% had pre-existing hypertension, 13% had baseline CKD, 3% gave history of chronic ethanol abuse, 3% had premorbid psychiatric illness, and 2% had chronic liver disease. Also, 34% of the cohort had at least one comorbid illness.
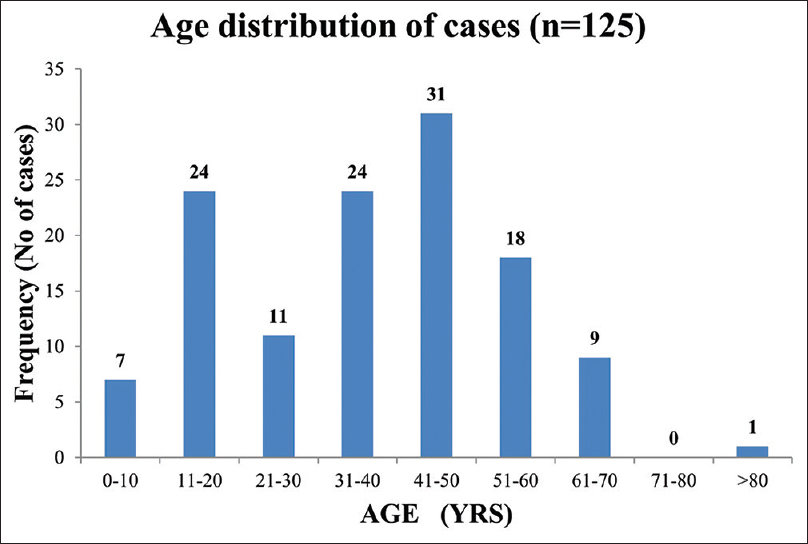
- Age distribution of IRGN cohort. IRGN = infection-related glomerulonephritis
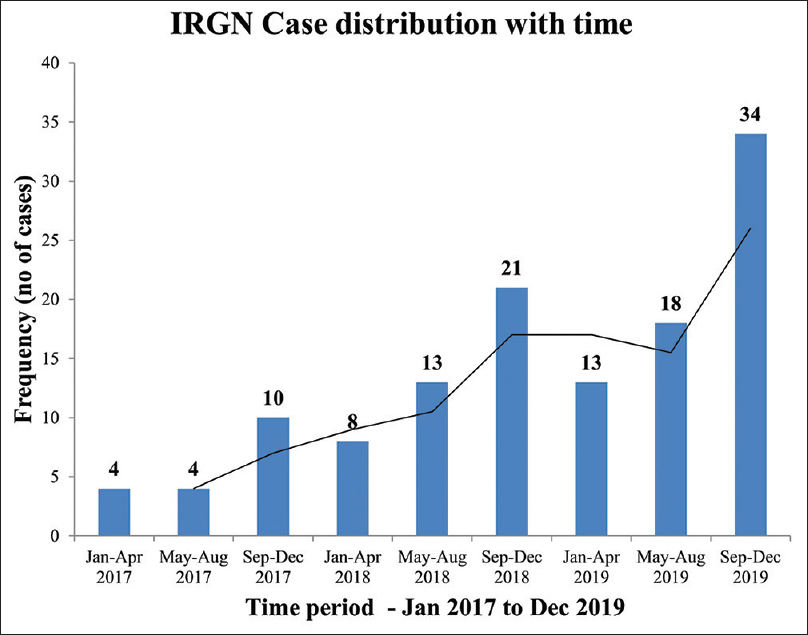
- Case distribution over time periods
Clinical presentation
Patients were stratified into one of four presentations – acute nephritis, nephrotic syndrome (NS), RPGN or RPGN on CKD. A majority (59%) of the cohort presented with acute nephritis. RPGN was seen in 30%, and RPGN complicated background CKD in 6%. NS was rare (5%) [Figure 3]. Patients with RPGN were likely to be older (mean 45 years) and male (53%) in comparison to those with acute nephritis (mean 31 years; 38% male). Those who presented with RPGN were more likely to have diabetes (36% vs. 6%; P < 0.001), baseline hypertension (30% vs. 8%; P = 0.001), and background CKD (23% vs. 6%; P = 0.01) in comparison to those with acute nephritis [Figure 4]. More than half the RPGN cohort (55%) had an identifiable comorbidity at presentation, compared to 22% in the acute nephritis group (P < 0.001).
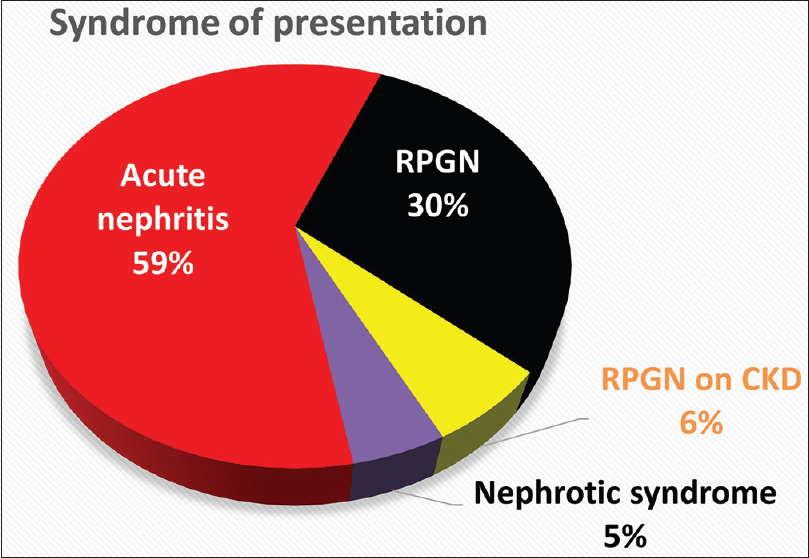
- Syndrome of presentation
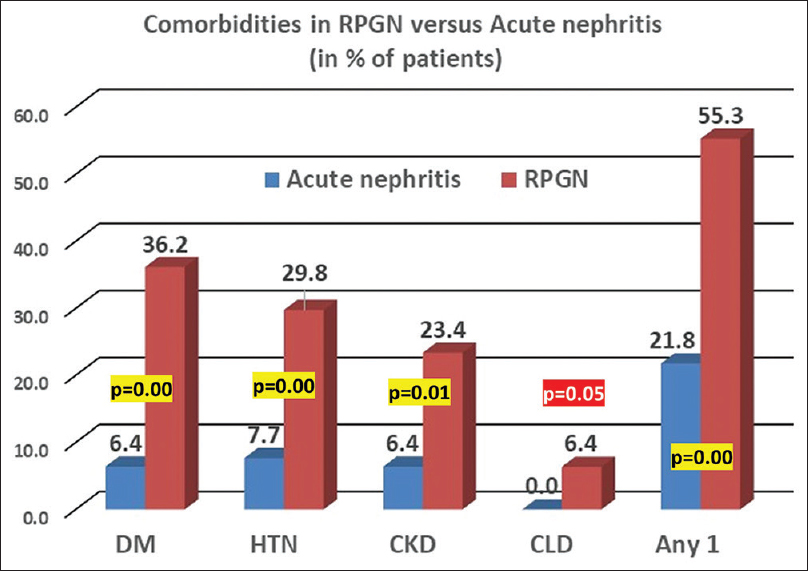
- Comorbidities in RPGN versus acute nephritis sub-cohort. CKD = chronic kidney disease, CLD = chronic liver disease, DM = diabetes mellitus, HTN = hypertension, RPGN = rapidly progressive glomerulonephritis
Presentation was typically acute with the median duration of symptoms being 7 days (interquartile range [IQR] = 7). Also, 92% had peripheral edema at presentation, while macrohematuria was seen in only 28%. Oliguria was a symptom in 52%, with documented AKI in 78% of patients. Dyspnea was prominent in 40% of the cohort. Hypertension, defined as more than 140/90 mmHg in adults or more than the 95th centile in pediatric patients, was seen in 80%. Results showed that 45% had a peak systolic blood pressure of more than 160 mmHg and 6% had more than 200 mmHg. Also, 18% needed intensive care admission for need of ventilatory support or management of hypertensive emergency [Table 1].
| n=125 | % | |
|---|---|---|
| Mean age in years (SD) | 37 (17.6) | |
| No. of females | 74 | 59 |
| Diabetes mellitus | 22 | 18 |
| Hypertension | 20 | 16 |
| CKD | 16 | 13 |
| Previous psychiatric illness | 4 | 3 |
| Symptom duration (days – median) | 7 (IQR 7) | |
| Macrohematuria | 35 | 28 |
| Dyspnea | 50 | 40 |
| Oliguria | 65 | 52 |
| Edema | 115 | 92 |
| HTN (BP >140/90) | 100 | 80 |
| SBP >200 | 7 | 6 |
| SBP >180 | 16 | 13 |
| SBP >160 | 56 | 45 |
| AKI | 97 | 78 |
| RRT requiring | 30 | 24 |
| ICU stay | 22 | 18 |
CKD: Chronic kidney disease, HTN: Hypertension, ICU: Intensive care unit, RRT: Renal replacement therapy, SBP: Systolic blood pressure, SD: Standard deviation
Laboratory evaluation
The mean peak serum creatinine at presentation was 3.6 (±3.2) mg/dl. Creatinine more than 4 mg/dl was seen in 34% of patients. Microscopic hematuria was universal; red blood cell casts were detected in 31%; and leukocyturia was noted in 32%. The median proteinuria was 1.9 g/day with a mean serum albumin of 3.2 (±0.5) g/dl. Nephrotic range proteinuria was seen in 33% of patients. Low serum complement was noted in 65% of the patients, of which 52% had low C3 alone and 13% had low C3 and C4. None had isolated C4 depression. Also, 35% of the cohort had normal complement levels [Figure 5].
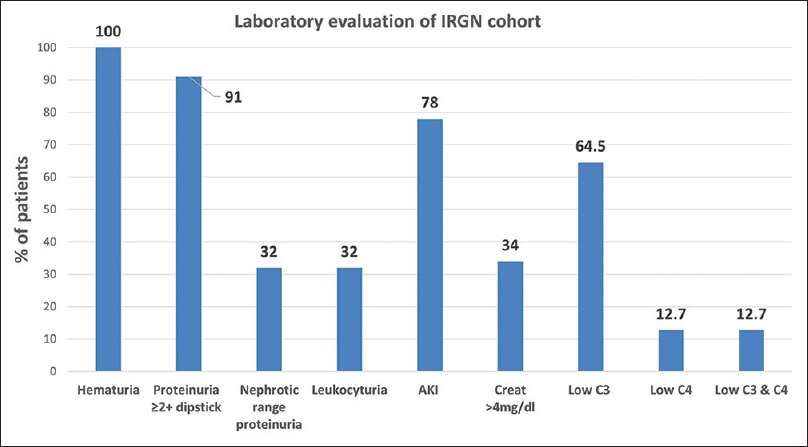
- Laboratory evaluation
Diagnosis
Thirty-nine patients (31%) were diagnosed based on clinical and laboratory criteria as they fulfilled the definition of “typical IRGN.” Eighty-six of 125 cases (69%) needed a kidney biopsy because of atypical features. RPGN presentation (49%) was the most common indication for biopsy, followed by atypical complement profile (31%), absence of clear focus of infection (10%) and high creatinine at the end of 10 days (10%). EM was needed for clarification of diagnosis in five patients in the screening cohort, of which three were excluded from the final cohort in favor of a diagnosis of C3 glomerulopathy.
Infection source/microbiology
The diagnosis of infection source was made by a combination of clinical assessment and radiological, microbiological, and serological evaluation. The most common site of infection was skin and soft tissue (31%), followed by pharyngitis/tonsillitis in 20%. Atypical sources of infection included otomycosis, endophthalmitis after cataract surgery, wisdom tooth abscess, osteomyelitis, pancreatitis with sepsis, and scrub typhus. We had two patients of dengue fever who developed worsening hypertension, edema, azotemia, and active sediments in urine and were diagnosed with IRGN. Also, 28% of patients who presented had no discernible focus of infection even after extensive evaluation [Figure 6].
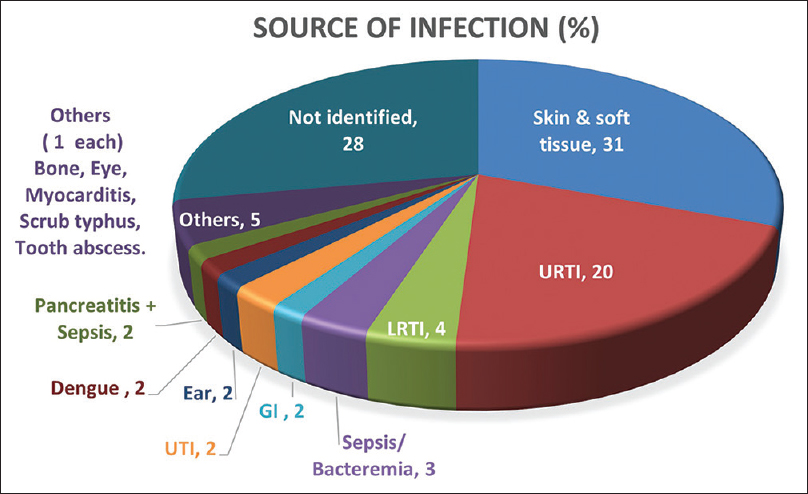
- Infection source. GI = gastrointestinal, LRTI = lower respiratory tract infection, URTI = upper respiratory tract infection, UTI = urinary tract infection
Microbiological confirmation was possible in only 42% of cases. Streptococcus was the most commonly identifiable etiology, followed by staphylococci and gram-negative bacteria. Aspergillus was identified in the KOH mount of ear scrapings in the patient diagnosed with otomycosis. No organism was isolated in 58% of this group [Table 2].
| Organism | No of cases | % |
|---|---|---|
| Streptococcus | 31 | 25% |
| Staphylococcus (MSSA) | 9 | 7% |
| CONS | 2 | 1.6% |
| MRSA | 1 | 0.8% |
| E. Coli | 3 | 2.4% |
| GNB bacteremia | 2 | 1.6% |
| Dengue virus | 2 | 1.6% |
| Scrub Typhus | 1 | 0.8% |
| Aspergillus | 1 | 0.8% |
| None identified | 73 | 58% |
IF: Immunofluorescence
Histopathology
Eighty-six patients were subjected to a renal biopsy. All biopsies were done with 18-gauge biopsy guns; tissue was adequate for diagnosis in all specimens. No major biopsy complications were encountered in any of the patients. IF and LM were done on all biopsy samples. Figure 7 is a collage of representative biopsy images from one of our cases. C3 (mean intensity, 2.9) was the universal immune reactant, with IgG (mean intensity when positive, 1.7) being the most common immunoglobulin (77%) and IgA being the least common (5%). C3 was the only immune reactant in 23% and dominant by at least two grades in 57% of biopsies. C1q (mean intensity when positive, 1.3) was deposited in 21% of biopsies and was always weaker than C3. Complete full house IF was not seen, but full house except IgA was seen in six cases (7%) [Table 3 and Table 4]. C3 dominance was associated with the need for renal replacement therapy (RRT), progression to CKD (P = 0.04), and development of end-stage renal disease (ESRD; P = 0.01) in univariate analysis.
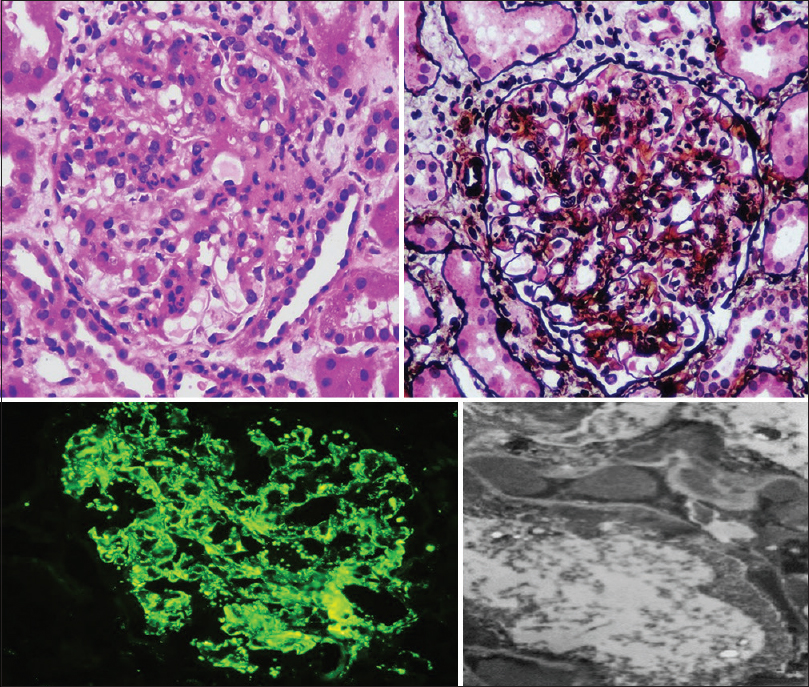
- Clockwise from top left. Endocapillary proliferative and exudative glomerulonephritis in hematoxylin and eosin stain (400×) and periodic acid–methenamine silver stain (400×). Electron microscopy showing subepithelial humps. Extensive granular capillary wall and mesangial staining of C3 on IF. IF = immunofluorescence
| Immune reactant | No. of biopsies (n=86) | % of biopsies |
|---|---|---|
| C3 | 86 | 100% |
| C1q | 18 | 21% |
| IgG | 66 | 77% |
| IgM | 8 | 9% |
| IgA | 4 | 5% |
| Immune pattern | No. of biopsies (n=86) | % of biopsies |
|---|---|---|
| Only C3 | 20 | 23% |
| C3+IgG | 66 | 77% |
| C3 ≥2+ dominant | 49 | 57% |
| IgG dominant | 0 | 0% |
| IgG/C3 co-dominant | 10 | 12% |
| IgA dominant | 4 | 5% |
| Full house | 0 | 0% |
| Full house other than IgA | 6 | 7% |
IF: Immunofluorescence
There were four cases (5%) of IgA-dominant IRGN. All were female, with a mean age of 52 years. Three of these patients had crescents more than 20% on biopsy and they progressed to CKD. One patient had normal renal function at presentation and continues to do so.
Twelve (±6) glomeruli were identified on average in the LM core. Endocapillary proliferative pattern was seen in 91% of all biopsies, mesangial proliferative pattern in 7%, and Membrano-proliferative glomerulonephritis in 2%. Though seven biopsies showed lobular accentuation, five did not qualify for MPGN pattern in the absence of basement membrane changes (double contours). Exudative pattern (neutrophilic infiltration) was seen in 80%. Crescentic IRGN defined by >50% of glomeruli showing crescents was rare (5%). Crescent in more than 20% of glomeruli was seen in 14%. Wire loop lesions were seen in one biopsy. Necrotizing lesions were not found [Table 5].
| No. | % of biopsies | |
|---|---|---|
| Histopathology | ||
| Endocapillary proliferative pattern | 78 | 91% |
| Mesangial proliferative pattern | 6 | 7% |
| MPGN | 2 | 2% |
| Crescentic IRGN (>50%) | 4 | 5% |
| Crescent more than 20% G | 12 | 14% |
| Neutrophilic infiltration (exudative) | 69 | 80% |
| Capillary loop obliteration | 35 | 41% |
| Lobular accentuation | 7 | 8% |
| Interstitial inflammation | 43 | 50% |
| Plasma cell infiltration | 3 | 3% |
| Eosinophil infiltration | 3 | 3% |
| Interstitial edema | 15 | 17% |
| Acute tubular injury | 51 | 59% |
| RBC casts | 25 | 29% |
| Global glomerulosclerosis >20% | 21 | 25% |
| Global glomerulosclerosis >50% | 7 | 8% |
| IFTA more than 10% | 17 | 20% |
| Coexistent pathology | ||
| Diabetic nephropathy | 8 | 9.3% |
| HTN arteriosclerosis | 6 | 7% |
| IgA Nephropathy | 1 | 1% |
| Chronic interstitial nephritis | 1 | 1% |
HTN: Hypertension, IRGN: Infection-related glomerulonephritis, LM: Light microscopy, RBC: Red blood cell
Interstitial cellular infiltrate was noted in 50%, with significant edema in 17%. Acute tubular injury was diagnosed in 59%. Atypical cell infiltration (plasma cell 3%, eosinophil 3%) was noted in some. Global glomerulosclerosis >20%, interstitial fibrosis and tubular atrophy (IFTA) >10%, and crescents >50% were each associated with ESRD in univariate analysis (P < 0.02 for all). IFTA >10% was the only independent predictor of ESRD in binomial logistic regression (odds ratio [OR] 30.9).
IRGN is known to coexist with other renal pathologies. Eight biopsies showed features of diabetic nephropathy (one RPS class 4; rest RPS class 2/3) with varying degrees of arteriosclerosis and arteriolar hyalinosis. Six additional biopsies showed features of moderate arteriosclerosis. There was one biopsy which showed features of IRGN superimposed on chronic interstitial nephritis and another with coexisting IgA nephropathy. Among all diabetics who underwent renal biopsy for any indication at our institute over the past 3 years, IRGN represents the most common pathology other than diabetic nephropathy [Table 6].
| Diagnosis | % |
|---|---|
| Diabetic nephropathy | 57% |
| IRGN | 29% |
| Acute Tubular Injury | 16% |
| Moderate/severe arteriosclerosis | 7% |
| ATIN | 5% |
| Membranous nephropathy | 5% |
| Collapsing Glomerulopathy | 4% |
| MPGN | 2% |
| IgAN | 2% |
| Acute pyelonephritis | 2% |
| Malignant hypertension | 2% |
IRGN: Infection-related glomerulonephritis,
The renal allograft biopsy with IRGN showed C3 3+ and IgG 1+ deposits on IF. LM revealed endocapillary proliferative and exudative GN with interstitial inflammation and edema (ptc1; i2).
Treatment and outcomes
Fourteen patients (11%) received pulse and/or maintenance steroids. There was no difference in outcomes in terms of need for RRT, CKD/ESRD progression, or mortality between the groups treated and not treated with steroids. Nearly one-fourth (24%) of the IRGN cohort required RRT (hemodialysis -HD). The most common and prominent indication for hemodialysis initiation was fluid overload with pulmonary edema. Follow-up data was available for 93% of the cohort. Mean duration of follow-up was 203 (range 102–933) days. CKD progression was defined as estimated glomerular filtration rate (eGFR) <60 ml/min and/or proteinuria >1 g/day persistently after 3 months. In patients with baseline CKD (Stage 2/3), progression was defined as development of Stage 4/5 CKD or ESRD. The overall progression to CKD in the cohort was 33%, with 69% of the RPGN sub-cohort progressing. Age more than 50 years, RPGN presentation, and CKD at baseline were the independent risk factors for CKD progression in logistic regression.
ESRD developed in 12% (15 patients) of the IRGN cohort, and the overall mortality was 10% (12 deaths). Increasing age, RPGN presentation, diabetes, baseline CKD, and need for dialysis were strongly associated with ESRD development in univariate analysis. Diabetes stood out as an independent risk factor for ESRD by logistic regression. Diabetes and need for hemodialysis also independently predicted mortality in multivariate analysis. IRGN-RPGN portended a poor prognosis, with ESRD developing in 31% and death occurring in 22% of this sub-cohort. Figure 8 compares the outcomes in the larger IRGN cohort (all cases) and the RPGN subgroup.
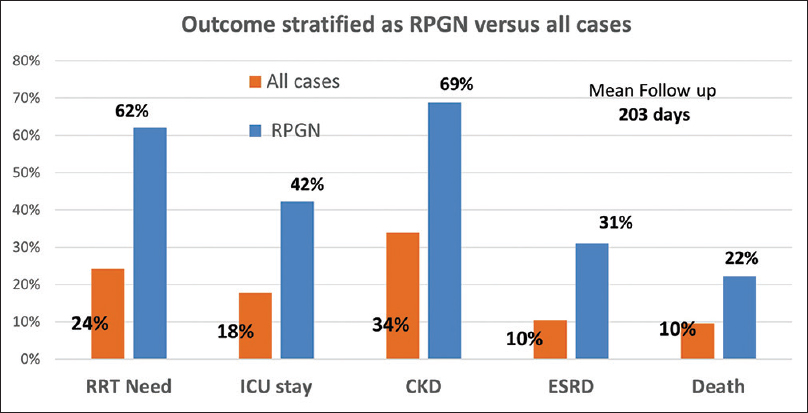
- Outcomes. CKD = chronic kidney disease, ESRD = end-stage renal disease, ICU = intensive care unit, RPGN = rapidly progressive glomerulonephritis, RRT = renal replacement therapy.
In the short term, two deaths occurred due to direct complications of hemodialysis during the acute illness (sudden cardiac death during hemodialysis- 1, air embolism- 1). Three patients died due to underlying illness (hepatocellular carcinoma- 1, viral myocarditis/sepsis- 1, diabetic foot/sepsis- 1). Four deaths occurred on maintenance dialysis elsewhere. Two patients on dialysis at our center and another patient who presented with acute nephritis and was discharged in a stable condition died at home, with the cause not identified.
Adult versus pediatric analysis
We analyzed the cohort as two subgroups – pediatric, defined as <18 years of age, and adults. The pediatric cohort experienced milder disease, usually with an acute nephritic presentation and benign clinical outcomes. Oliguria, AKI, and peak creatinine were significantly lower in the pediatric group. Only one-third of the pediatric group required biopsy in view of atypical IRGN, in contrast to 80% of the adult cohort. No major differences were made out in histopathology, except for higher grades of chronicity in adult biopsies. All pediatric patients had an identifiable source of infection, usually remote/recent, usually not concurrent. This is in contrast to 41% of the adult group in whom no focus was identified and 28% who had an active focus of infection at the time of diagnosis of IRGN. Adverse outcomes including CKD progression and ESRD were significantly higher in the adult population. There was a trend toward higher mortality in the adult cohort as well. This comparison is presented in Table 7.
| Parameter | Pediatric | Adult | P |
|---|---|---|---|
| No. of cases | 28 | 97 | |
| Mean age, years (SD) | 12 (4.5) | 44 (12.7) | 0.00 |
| Female | 39% | 66% | 0.02 |
| RPGN | 14% | 43% | 0.01 |
| Gross hematuria | 46% | 23% | 0.03 |
| Oliguria | 25% | 60% | 0.00 |
| AKI | 57% | 83% | 0.01 |
| Mean creatinine mg/dl (SD) | 1.7 (1.1) | 4 (3.3) | 0.00 |
| Creatinine>4 mg/dl | 10% | 41% | 0.00 |
| Low C3 | 78% | 62% | 0.16 |
| Low C4 | 22% | 10% | 0.09 |
| Normal C3 and C4 | 22% | 38% | 0.16 |
| Biopsy need | 29% | 80% | 0.00 |
| C3 deposition | 100% | 100% | 1.00 |
| C3 2+dominance | 50% | 57% | 0.73 |
| IgA dominance | 0% | 5% | 1.00 |
| ECP | 100% | 92% | 0.08 |
| Mesangial proliferation | 50% | 22% | 0.10 |
| Crescent>20% | 0% | 17% | 0.35 |
| Global GS>10% | 0% | 37% | 0.05 |
| IFTA | 0% | 22% | 0.20 |
| No infection focus identified | 0% | 33% | 0.00 |
| RRT requiring | 4% | 28% | 0.00 |
| CKD progression | 4% | 42% | 0.00 |
| ESRD | 0% | 15% | 0.04 |
| Death | 0% | 12% | 0.07 |
CKD: Chronic kidney disease, ESRD: End-stage renal disease, RPGN: Rapidly progressive glomerulonephritis, RRT: Renal replacement therapy, SD: Standard deviation, ECP=endocapillary proliferation
Discussion
IRGN represents 24% of all renal biopsies done at our center over the past 3 years. It is an important cause for nephrology referrals and admissions. The female preponderance in the adult cohort in a ratio of nearly 2:1 is striking. This is different from previous literature, where there is either a male dominance or equivalence between both sexes.[34] Males, however, had a higher risk for RPGN presentation (OR 2.83) and RRT need (OR 4.13) (P < 0.01). We have also described a clustering of cases in the last 4 months of the year, where there typically occurs an “epidemic” of IRGN. The month of September as the peak season of IRGN has been alluded to by other publications.[2] Variations in seasonal susceptibility to infections including streptococci might explain this phenomenon.[5]
The adult cohort had more severe disease at presentation and a higher chance of adverse outcome than the pediatric one. However, RPGN presentation (4 cases) occurred even in the latter. Though only one child had dialysis requiring renal failure, five required intensive care unit (ICU) admission in view of hypertensive emergency (pulmonary edema, encephalopathy). Thus, even in children, there is a risk for serious morbidity in IRGN.[6] In contrast to children, 80% of adults in our cohort required a biopsy in view of atypical features. The common differential diagnoses considered at the time of biopsy included IgA nephropathy and lupus nephritis.
IgA nephropathy is usually a clear-cut diagnosis, with IgA as the most dominant or codominant immune reactant on IF, where C3, if present, is usually weaker than IgA. The two caveats here include identifying coexistent IRGN with IgA nephropathy and IgA-dominant IRGN. We had one patient with chronic liver disease and an RPGN presentation, whose biopsy revealed endocapillary proliferation and strong C3 and IgG deposits over the capillary loops, with IgA 3 + deposits localized over the mesangium. He had evidence of streptococcal infection with ASO titers of 1500 IU/l. This probably represents an IRGN superimposed on secondary IgA nephropathy. It is important to differentiate this from an IgA-dominant IRGN (IgA-IRGN), where IgA, C3, and IgG colocalize to the capillary loop and/or mesangium. IgA is the dominant or sole immunoglobulin, with C3 being typically stronger in staining than IgA.[1]
We had four cases of IgA-IRGN, three of whom had no previous comorbidities. One patient had diabetes and obesity. This is consistent with emerging literature that this entity is increasingly diagnosed in non-diabetics.[78] In our cohort, one patient had streptococcal infection and another had post-cataract ocular infection; no infection was identified in the other two cases. Most series of IgA-IRGN reported in literature note the occurrence of Methicillin resistant staphylococcus aureus (MRSA) infections and serious gram-negative bacillary foci.[910]
Lupus nephritis is clinically differentiated by pertinent serology and other extrarenal manifestations. Low C3 and C4 is a hallmark of lupus activity, hence considered an indication for biopsy in this study. But even in our IRGN cohort, 13% of patients had depressed serum C4 levels. None of these patients had serological or clinical evidence of lupus. Additional clues to lupus on the biopsy include the deposition of c1q, characteristically in a full house pattern, which may be seen occasionally in IRGN. We did not identify full house IF on any of our biopsies. However, full house excluding IgA was seen in six biopsies (7%) and C1q deposition was prominent in 18 (21%). IRGN is an immune complex–mediated disease in which there is some evidence of early transient classical complement pathway activation.[11] In the early phase, there is deposition of immune complexes (and complement components) in the subendothelial space, as hypothesized by Nadasdy et al.[12] The clearance of this immune complex lattice by phagocytes and subsequent breakdown of the lattice activates the alternate pathway of complement, leading to heavy C3 deposition. Thus, C3 deposition, being a later event, is usually stronger in staining to either IgG or c1q.[1314] IgG (absent in 24% of our biopsies) or c1q (absent in 80%) may be completely cleared out and, therefore, negative. In our biopsies with c1q deposition, c1q was two grades (12/17) or one grade (5/17) weaker than C3. Lupus may be more likely when the IgG, C3, and c1q staining are all strongly positive.[15]
C3 glomerulopathy is another close differential on biopsy. Persistently low serum complements at 6–8 weeks suggests continuing complement dysregulation. Forty percent of C3 glomerulopathies (C3G) can be preceded by infectious triggers and 57% show elevated ASO titers.[16] On LM, it may be difficult to differentiate both entities due to overlapping features. Though classically, a membranoproliferative pattern is described in C3G, endocapillary or mesangial proliferation can occur. By definition, C3 2+ dominance and weak or absent staining for other immune reactants are required for a diagnosis of C3G (seen in 22% of our biopsies). Therefore, in doubtful cases, EM may be contributory. Predominance of subendothelial and mesangial deposits is more suggestive of C3G. Despite the association of subepithelial humps classically with IRGN, evidence is now emerging on its occurrence in C3G as well; preferential localization to the mesangial waist and evidence of resorption of deposits may suggest IRGN.[1718]
We needed clarification of diagnosis between IRGN and C3G in five cases for which EM was employed. One such case was of a 12-year-old child with acute nephritis preceded by acute pharyngitis. In view of a similar history at 2 years of age, he underwent a renal biopsy at this visit. The biopsy showed endocapillary and mesangial proliferation without exudation. C3 3+ was the sole reactant on IF. EM was done, which showed subendothelial and mesangial deposits, suggestive of C3G. He, however, had normalization of serum C3 at 8 weeks, and on 3-year follow-up, he has normal renal function with no proteinuria or hematuria or recurrence. Therefore, the distinction between IRGN and C3 glomerulopathy is often clinical.
The concept of atypical IRGN was introduced by Sethi et al.[19] and complement dysregulation may be underlying it. A significant number (22%) had isolated C3 deposition on the biopsy in our series. Though all of them were clinically consistent with a diagnosis of IRGN, the known clinical and histological overlap between IRGN and C3G suggests that some of these atypical cases perhaps need to be screened for defects in the complement regulatory genes. Cost factors and therapeutic limitations are major hurdles in this approach.
IRGN is the most common nondiabetic renal pathology identified in our cohort of diabetic biopsies over 3 years. This contrasts with most other series where IgA nephropathy, membranous nephropathy, and Focal and Segmental Glomerulosclerosis (FSGS) occupy the top spot in a pooled meta-analysis of diabetic renal biopsies worldwide.[20] Diabetes is also an important risk factor for progression to ESRD and mortality in our IRGN cohort. Moreover, among diabetic nephropathy patients, the occurrence of IRGN is often the tipping point for progression to ESRD.[21]
A clinical source of infection was identifiable in 72% of the cohort. We identified atypical sites of infection, which is possible with focused evaluation. We report dengue virus–related IRGN as the possible cause of acute nephritis in two of our patients. Though traditionally, bacterial pathogens have been implicated, other atypical organisms that have been identified as the foci of infection in IRGN include varicella, herpes zoster, and scabies.[4] Table 8 lists the evaluation that may be undertaken for a patient with IRGN.
| Serological workup |
|---|
| Serum C3, C4 levels |
| ANA by IF |
| p-ANCA, c-ANCA |
| Infection focus workup |
| Detailed history |
| Physical examination – focus on skin and soft tissue |
| ENT assessment/throat swab |
| Dental assessment |
| Echocardiography for vegetations |
| ASO titers, anti-DNaseB |
| Blood culture, urine routine, urine culture |
| Stool for parasites |
| Ultrasound abdomen and pelvis |
| Chest X-ray/alternate chest imaging |
| Focused evaluation: abscess drainage+pus c/s |
IF: Immunofluorescence, IRGN: Infection-related glomerulonephritis, ANCA: Anti neutrophilic cytoplasmic antibody
The adverse outcome profile that we have demonstrated in this cohort suggests that IRGN may be an important contributing factor for ESRD with no disease-modifying therapy at present. Better understanding of the pathophysiology, recognition of underlying complement dysregulation, and early identification and treatment of infection are prudent.[22]
Conclusion
Adult-onset IRGN is characterized by poor outcomes; pediatric cases, though usually benign, can still manifest with life-threatening complications. Many adult cases are atypical in nature with RPGN presentation, normal complement profile, or with no identifiable infectious focus. Histopathology is important in the diagnosis, and subtle differences in IF and LM may provide clues to the right diagnosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013;83:792-803.
- [Google Scholar]
- Follow-up study of post-infectious glomerulonephritis in adults: Analysis of predictors of poor renal outcome. Saudi J Kidney Dis Transplant. 2014;25:1210-6.
- [Google Scholar]
- The current spectrum of infectious glomerulonephritis. Experience with 76 patients and review of the literature. Medicine (Baltimore). 1995;74:63-73.
- [Google Scholar]
- The epidemiology, clinical features, and outcome of infection-related glomerulonephritis from East India: A single center experience. Indian J Nephrol. 2017;27:307-12.
- [Google Scholar]
- Epidemiology of group A streptococcal pharyngitis & impetigo: A cross-sectional & follow up study in a rural community of northern India. Indian J Med Res. 2009;130:765-71.
- [Google Scholar]
- Long-term prognosis of diffuse proliferative glomerulonephritis associated with infection in adults. Nephrol Dial Transplant. 2002;17:1204-11.
- [Google Scholar]
- IgA-dominant infection-related glomerulonephritis in India: A single-center experience. Indian J Nephrol. 2017;27:435-9.
- [Google Scholar]
- Immunoglobulin A-dominant postinfectious glomerulonephritis: Frequent occurrence in nondiabetic patients with Staphylococcus aureus infection. Hum Pathol. 2011;42:279-84.
- [Google Scholar]
- The features in IgA-dominant infection-related glomerulonephritis distinct from IgA nephropathy: A single-center study. Clin Exp Nephrol. 2018;22:1116-27.
- [Google Scholar]
- Use of steroid therapy in immunoglobulin A-dominant poststaphylococcal glomerulonephritis. Hong Kong J Nephrol. 2015;17:46-9.
- [Google Scholar]
- Post-streptococcal acute glomerulonephritis in children: Clinical features and pathogenesis. Pediatr Nephrol. 2011;26:165-80.
- [Google Scholar]
- Infection-related glomerulonephritis: Understanding mechanisms. Semin Nephrol. 2011;31:369-75.
- [Google Scholar]
- Complement activation in acute glomerulonephritis in children. Int J Pediatr Nephrol. 1985;6:17-24.
- [Google Scholar]
- Complement components and complement activation in acute poststreptococcal glomerulonephritis. Int Arch Allergy Appl Immunol. 1979;58:274-84.
- [Google Scholar]
- The clinicopathology and outcome of post-infectious glomerulonephritis: Experience in 36 adults. J Med Assoc Thail Chotmaihet Thangphaet. 2006;89(2):S157-62.
- [Google Scholar]
- Infection-related glomerulonephritis and C3 glomerulonephritis-Similar yet dissimilar: A case report and brief review of current literature? Cureus. 2020;12:e7127. doi: 10.7759/cureus. 7127
- [Google Scholar]
- C3 Glomerulopathy: Clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol CJASN. 2014;9:46-53.
- [Google Scholar]
- C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 2018;93:977-85.
- [Google Scholar]
- Atypical post-infectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83:293-9.
- [Google Scholar]
- Renal biopsy in patients with diabetes: A pooled meta-analysis of 48 studies. Nephrol Dial Transplant. 2017;32:97-110.
- [Google Scholar]
- The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8:1718-24.
- [Google Scholar]
- Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol. 2011;22:187-95.
- [Google Scholar]







