Translate this page into:
Longitudinal Study to Find the Association of Serum Phosphorus Level with FGF23 in Three Different Hyperphosphatemia Management Groups of Stage 3 and 4 Chronic Kidney Disease (CKD) Patients
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
There is paucity of clinical evidence on target serum phosphorus levels in early chronic kidney disease (CKD). Present longitudinal study was done to find target phosphorus level and its association with fibroblast growth factor (FGF23) in three different hyperphosphatemia management groups.
Methods:
This 1-year, prospective, randomized controlled, open-labelled study was conducted among three equally allocated treatment groups that consisted of 120 screened early CKD patients totally. Group 1 patients were given dietary phosphorus modification (n = 40), group 2 patients were administered calcium-based phosphate binders (n = 40), and group 3 patients were given non–calcium-based phosphate binders (n = 40). Three-monthly dietary assessment, MDRD estimated glomerular filtration rate (eGFR), phosphorus, calcium, iPTH, alkaline phosphatase, and six-monthly FGF23, 2D echocardiography, and X-ray of chest and abdomen were performed. Association of three categories of phosphorus level up to 3.9, 4–5, and >5mg/dl, rate of progression of all parameters, and correlation with FGF23 were studied among all three groups.
Results:
At baseline, all clinical and biochemical parameters were equally distributed with a controlled nutritional phosphate among all groups. There was no significant difference of FGF23 levels from all the three categories of phosphorus level among all groups. Serum phosphorus at the level of 5 mg/dl was associated with iPTH and eGFR at 1 year. Over 1 year, there was a significant decline in serum phosphorus levels in group 1 (P 0.02), group 2 (P 0.00), and group 3 (P 0.05). FGF23 declined significantly only in group 3 (P 0.00). There was no correlation of FGF23 with serum phosphorus levels (P 0.13). However, FGF23 correlated positively with iPTH (P 0.03, r = 0.19)
Conclusion:
Serum phosphorus levels upto 5mg/dl had no effect on FGF23 at early CKD stages. Although different treatment groups showed significant phosphorus reduction, non-calcium phosphate binder had a major impact on FGF23 reduction.
Keywords
FGF23
hyperphosphatemia management
phosphorus in early CKD
Introduction
Chronic kidney disease (CKD) is a global public health problem affecting 10%–16% of the world population with the stage-wise prevalence being stage 1, 13.4%; stage 2, 9.9%; and late stages (3–5), 4.5%.[12] Mineral bone disease develops at stage 3 due to impaired conversion of 25-(OH)D to 1,25-(OH)2D, reducing intestinal calcium absorption and increasing parathyroid hormone (PTH) level. Along with PTH, fibroblast growth factor (FGF23) reduces renal tubular reabsorption of filtered phosphorus in response to changes in gastrointestinal phosphorus uptake, thereby preventing major changes in serum phosphorus levels.[3] As CKD advances, there is progressive deterioration in mineral homeostasis, leading to hyperphosphatemia, hypocalcemia, hyperparathyroidism, and increase in FGF23. At this stage, composite measurements of serum phosphorus and FGF23 may become an integral and improved approach in therapeutic decision-making.[4]
It is unclear whether actual serum phosphorus levels and other markers of disordered bone and mineral metabolism (e.g., serum calcium, calcium–phosphorus product) are associated with progression of kidney disease in patients with CKD. Limited data suggest that phosphate retention may cause harm in the pre-dialysis stages. There is a huge gap with regard to prospective clinical trials assessing the effect of phosphate-lowering strategies on clinically meaningful endpoints. As per KDIGO 2017, it has been suggested that in patients with CKD G3a to G5D, individual values of serum calcium and phosphate, evaluated together, be used to guide clinical practice.[5] Further, in these patients, it is suggested that phosphorus has to be reduced toward normal range, but the exact value and its benefit are unclear (2C).[5] Hence, the present 1-year longitudinal study was planned to find the association of serum phosphorus level with FGF23 in three different hyperphosphatemia-treated CKD stage 3 and 4 patient groups.
Material and Methods
Study design
This observational study was a single-center, parallel arm, randomized, and open-labeled study performed during January 2019–August 2020 at our hospital after obtaining approval from the institute's ethics committee.
Inclusion and exclusion criteria
All consecutive CKD stage 3 and CKD stage 4 patients having serum phosphorus level between 3.5 and 7.0 mg/dl who gave their consent to participate were included in the study. Pregnant or breast feeding women patients were excluded.
Sample size
Based on Modification of Diet in renal Disease-1 (MDRD 1), all CKD 3 and 4 patients were divided into three equal groups as follows: group 1:only dietary modification; group 2:calcium-based phosphate binder; and group 3:non–calcium-based phosphate binders. The biomarker FGF23 was considered as a dependent variable; upto 50 pg/ml was considered as normal.[67] Further, 5% level of alpha error and 80% power with one-side test was taken. The sample size for each group was calculated as n = σ2 (Zα + Zβ) 2/e2 = 76.56 × (1.64 + 1.28)2/16 = 40 (for one group). As there were three groups, the total sample size calculated was 120; considering 25% dropout rate, a total of 150 patients were included.
Screening and randomization
All CKD patients of age more than 18 years attending the outpatient department were screened for serum creatinine to determine the estimated glomerular filtration rate (eGFR) and serum phosphorus levels, keeping in mind the inclusion and exclusion criteria of the study. Out of 330 screened patients, a total 150 patients were randomized initially, but only 120 patients completed 1 year. These patients were subjected to computer-based randomization with an allocation ratio of 1:1:1 in threegroups equally [Figure 1] as follows: group 1(n = 40; normal 700–800 mg/day phosphorus diet): this group was subjected to normal phosphorus diet during thefirst 6 months, followed by low-phosphorus diet in the next 6 months; group 2 (n = 40) patients were given calcium-based phosphate binder (starting dose was tab calcium acetate 667mg BD) along with a low-phosphorus diet, that is, 500–700 mg/day; and group 3 (n = 40) patients were given non–calcium-based phosphate binder sevelamer (starting dose was 400 mg BD) with a low-phosphorus diet. Throughout the study, all patients were maintained on a constant dietary phosphorus intake by telephonic recall.
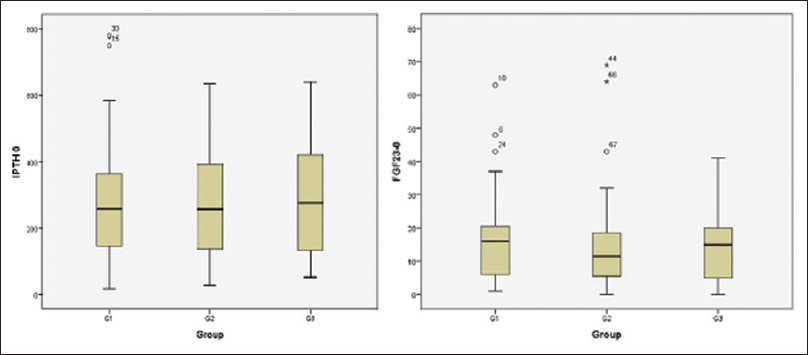
- Box plot showing mean iPTH/ FGF 23 at baseline among 3 groups
Definitions
-
Stages of CKD: Stage 1: eGFR ≥ 90 ml/min/1.73 m2; stage 2: 60–89 ml/min/1.73 m2; stage 3a: 45–59 ml/min/1.73 m2; stage 3b: 30–44 ml/min/1.73 m2; stage 4: 15–29 ml/min/1.73 m2; stage 5: <15 ml/min/1.73 m2 or end-stage renal disease.[5]
-
Bone disease in CKD: Presence of all the parameters including hyperphosphatemia (>5mg/dl), PTH >150pg/ml, FGF23 >50pg/ml, and vascular calcification was considered as bone disease.[56]
-
eGFR was calculated using the MDRD 1 formula as follows:[8] eGFR = 186 × (serum creatinine[mg/dl])−1.154 × (age [in years])−0.203 × (0.742 if female).
-
Hypertension (HTN): HTN was defined as per the JNC-8 guideline. Systolic blood pressure (SBP) ≥140 mmHg and diastolic blood pressure (DBP) ≥90 mmHg were used as the cut offs in patients with HTN, diabetes mellitus (DM), and CKD regardless of age and in adults younger than 60 years. In people of age 60 years or older, the cut off was equal to or more than 150/90.[9]
Data collection
At the beginning of the study, the demographic profile (all the patients were enquired about the name, age, gender, contact number, food habits, smoking and alcohol habits) and data on native kidney disease, chronic glomerulonephritis, diabetic nephropathy, hypertensive nephropathy, chronic tubulointerstitial disease, renal stone disease, obstructive nephropathy, cystic kidney disease, unknown, and comorbidities were noted.
Investigations
The investigations performed included complete blood count, urea, creatinine, phosphorus, calcium, iPTH, alkaline phosphatase (ALP), and MDRD-based eGFR. Apart from laboratory parameters, all participants underwent baseline 2D echocardiography to look for left ventricular hypertrophy, left ventricular ejection fraction (LVEF), and valvular calcification. X-ray abdomen was done to look for any evidence of prevertebral calcification and X-ray chest to evaluate the evidence of cardiomegaly. All these investigations were done at the beginning and thereafter three monthly till 1 year. FGF23 was measured at baseline and at 6 and 12 months only. iPTH was measured by third-generation immunoassay. For FGF23, samples were stored at −20°C after centrifugation (2000–3000 rpm) and were measured in our biochemistry laboratory using the enzyme-linked immunosorbent assay (ELISA) kits from Immutopics, Inc.
Follow-up
All the patients were also contacted telephonically to ensure the diet intake. All the investigations were performed every 3 months and 6 monthly (FGF23, 2D echocardiography, X-ray of chest and abdomen).
Statistical analysis
All statistical analyses were performed using the Statistical Software for Social Sciences (SPSS) Windows version 20.0. Quantitative data was expressed as mean and standard deviation. For finding the difference between more than two groups, analysis of variance (ANOVA) test or Kruskal–Wallis H test was used. Qualitative data was expressed as percentage. Repeated measure parametric and non-parametric one-way ANOVA was used to find the progression of clinical and biochemical parameters. Correlation and regression analyses were carried out between all parameters and FGF23. Based on FGF23, patients in all the groups were further stratified into category 1, 2, and 3 according to the serum phosphorus levels of up to 3.9, 4–5, and >5mg/dl, respectively. Chi-square test was used to find the percentage of cases in relation to FGF23 among all groups over a period of 1 year.
Results
Baseline demographic profile
The mean age of 120 patients who completed the 1-year study period was 52.51 ± 15.5 years and there was a slightly male preponderance (59.2%). All the clinical parameters were comparable among all three study groups [Table 1]. Majority of the patients were in CKD stage 3b and 4. Total protein and phosphorus intake were significantly higher in group 1, whereas total calories were the same. All the major biochemical and haematological parameters at baseline were comparable among the three groups, [Table 1]. The average serum calcium and phosphorus levels were reasonably well controlled and the PTH level was high in all the groups. The mean FGF23 levels were also similar among all groups [Figure 2].
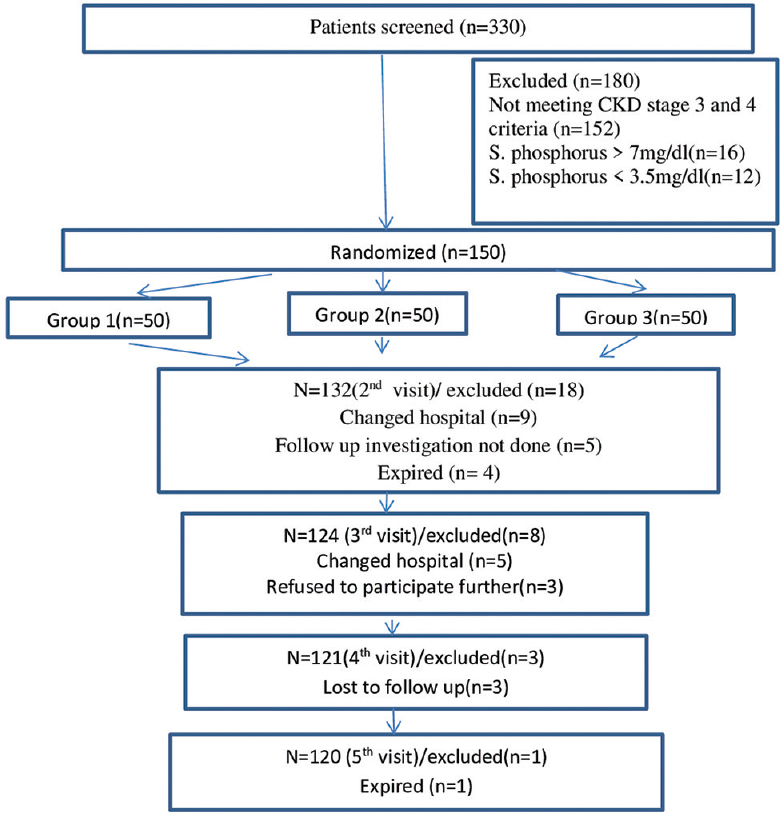
- Flow Diagram
| Parameters | Group 1 (n=40) Mean±SD/% |
Group 2 (n=40) Mean+SD/% |
Group 3 (n=40) Mean±SD/% |
P |
|---|---|---|---|---|
| Age (in years) | 54.50±13.41 | 51.93±16.26 | 51.10±16.80 | 0.60 |
| Mean BP (mm Hg) | 102.59±14.77 | 102.43±11.15 | 99.79±11.61 | 0.50*** |
| Gender (male) | 25 (62.5%) | 23 (57.5%) | 23 (57.5%%) | 0.87** |
| Smoking (Smoker) | 3 (7.5%) | 1 (2.5%) | 0 (0%) | 0.12 * |
| Alcohol (Alcoholic) | 1 (2.5%) | 1 (2.5%) | 0 (0%) | 0.70* |
| CKD Stage | ||||
| 3a | 15 (37.5%) | 7 (17.5%) | 12 (30.0%) | 0.206** |
| 3b | 7 (17.5%) | 14 (35.5%) | 13 (32.5%) | |
| 4 | 18 (45.0%) | 19 (47.5%) | 15 (37.5%) | |
| Diabetic’s | 15 (37.5%) | 16 (40.0%) | 12 (30.0%) | 0.624** |
| Hypertensive’s | 32 (80.0%) | 35 (87.5%) | 29 (72.5%) | 0.245** |
| H/o CVS disease | 3 (7.5%) | 5 (12.5%) | 2 (5.0%) | 0.601 * |
| H/o CVA | 0 | 2 (5.0%) | 1 (2.5%) | 0.772 * |
*Fisher’s exact test,**-Chi square, ***-kruskalwallis test, CKD- chronic kidney disease, CVS- cardiovascular disease, CVA- cerebrovascular disease. Data presented as Mean±SD or number (percentage)
Follow-up over 12 months
Group 1
On three-monthly follow-up over a period of 1 year, the renal function (urea, creatinine, and eGFR) and levels of serum potassium, total protein, albumin, and iPTH did not change significantly. There was progressive increase in the serum hemoglobin levels from the mean value of 10.75 ± 2.01 at baseline to 11.08 ± 1.49 at 12 months. There was a significant decrease in the mean value of serum phosphorus and alkaline phosphatase (ALP) from the 6th to 12th month. However, the calcium level decreased significantly at the 12th month. FGF23 increased significantly from 16.13 ± 13.44 at baseline to 29.17 ± 53.18 pg/ml at the 12th month (P-0.02). Both serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) were reduced all the time over 1 year. There was a statistically significant difference in phosphorus intake and protein intake at baseline [Table 2].
| Parameter | Total (n=120) Mean±SD/% |
GROUP 1 (n=40) Mean±SD/% |
GROUP 2 (n=40) Mean±SD/% |
GROUP 3 (n=40) |
P Mean±SD/% |
|---|---|---|---|---|---|
| Hb (gm%) | 52.51±15.49 | 10.75±2.01 | 10.98±1.76 | 10.80±1.86 | 0.91 |
| Blood Urea (mg/dl) | 61.98±19.62 | 66.73±21.66 | 59.20±19.67 | 61.75±16.93 | 0.41 |
| S. Creatinine (mg/dl) | 2.14+0.63 | 2.28±0.75 | 2.13±0.61 | 2.10±0.71 | 0.52 |
| Calcium (mg/dl) | 8.86±0.88 | 9.03±0.77 | 8.68±1.19 | 8.85±0.70 | 0.41 |
| Phosphorus (mg/dl) | 4.27±0.74 | 4.28±0.64 | 4.40±0.90 | 4.28±0.64 | 0.92 |
| e-GFR (ml/min) | 34.37±11.84 | 32.55±10.41 | 34.80±13.02 | 35.80±12.02 | 0.49 |
| Sodium (mmol/l) | 138.49±4.22 | 138.73±3.84 | 138.30±3.99 | 138.35±4.92 | 0.93 |
| Potassium (mmol/l) | 4.38±0.56 | 4.43±0.64 | 4.40±0.59 | 4.38±0.59 | 0.95 |
| SGOT (U/L) | 24.33±11.25 | 22.30±9.15 | 23.30±6.82 | 42.58±104.01 | 0.58 |
| SGPT (U/L) | 23.35±13 | 20.23±10.48 | 22.65±8.73 | 41.38±97.18 | 0.06 |
| T. Protein (gm/dl) | 7.29±0.74 | 7.30±0.61 | 7.38±0.90 | 9.45±13.89 | 0.56 |
| Albumin (gm/dl) | 4.01±0.55 | 4.18±0.59 | 4.08±0.42 | 3.88±0.56 | 0.06 |
| ALP (U/L) | 94.75±39.90 | 92.15±38.38 | 96.60±34.39 | 95.58±46.76 | 0.45 |
| iPTH (pg/ml) | 321.78±301.56 | 269.33±178.19 | 269.50±160.91 | 288.15±167.14 | 0.75 |
| FGF23(pg/ml) | 17.86±23.28 | 16.13±13.44 | 18.15±27.12 | 19.35±27.03 | 0.77 |
| X RAY Abdomen | |||||
| Vascular calcification | 1 (2.5%) | 1 (2.5%) | 1 (2.5%) | 1.000 * | |
| X RAY Chest | |||||
| Vascular calcification | 0.0% | 1 (2.5%) | 1 (2.5%) | 0.795* | |
| 2 D Echocardiography | 0.367* | ||||
| LVH | 13 (32.5%) | 11 (27.5%) | 7 (17.5%) | ||
| LVEF <60% | 3 (7.5%) | 7 (17.5%) | 3 (7.5%) | ||
| Valvular calcification | 2 (5.0%) | 2 (5.0%) | 1 (2.5%) |
Data presented as mean±SD. Group 1- dietary phosphorus restriction, group 2- calcium based phosphate binders, group 3- non calcium based phosphate binders. BP: Blood Pressure; SGOT: serum glutamic oxaloacetictransaminasee; SGPT: serum glutamic pyruvic transaminase; ALP- alkaline phosphatase; iPTH: intact parathyroid hormone; FGF23: fibroblast growth factor 23, LVEF: left ventricular ejection fraction. Computed using KRUSKAL WALLIS TEST.
Group 2
As in group 1, blood urea, serum creatinine, total protein, albumin, iPTH, and FGF23 levels did not change significantly. There was a statistically significant decrease in serum phosphorus in group 2 over the period of 12 months [Table 3]. It was also observed that there was a statistically significant increase in hemoglobin from 10.98 ± 1.76 g% at baseline to 11.70 ± 1.29 g% at 12 months (P < 0.0001). SGOT/SGPT decreased progressively over the period of 12 months. However, the nutritional parameter in terms of phosphorus-restricted diet was balanced over the period of study.
| Parameter | 0month Mean±SD |
3 months Mean±SD |
6month Mean±SD |
9 months Mean±SD |
12 month Mean±SD |
P | P (a, b, c) |
|---|---|---|---|---|---|---|---|
| HB (gm%) | 10.98±1.76 | 11.01±1.31 | 11.47±1.45 | 11.21±1.52 | 11.70±1.29 | <0.0001 | |
| Blood Urea (mg/dl) | 59.20±19.67 | 59.70±24.32 | 59.80±20.44 | 64.18±31.31 | 59.95±19.17 | 0.874 | |
| S. Creatinine (mg/dl) | 2.13±0.61 | 2.11±0.644 | 2.23±0.97 | 2.15±0.87 | 2.25±0.74 | 0.637 | |
| eGFR (ml/min) | 34.80±13.02 | 35.11±12.66 | 35.00±14.91 | 36.47±16.33 | 33.78±12.87 | 0.740 | |
| Calcium (mg/dl) | 8.68±1.19 | 8.95±0.72 | 8.70±0.85 | 8.87±0.67 | 8.63±0.63 | 0.199 | |
| Phosphorus (mg/dl) | 4.40±0.90 | 4.19±0.53 | 4.13±0.65 | 4.00±0.64 | 4.00±0.60 | 0.009c | c-0.009 |
| Sodium (mmol/L) | 138.30±4.00 | 138.0±3.14 | 139.25±3.44 | 139.48±3.74 | 139.88±3.46 | 0.078 | |
| Potassium (mmol/L) | 4.40±0.59 | 4.34±0.42 | 4.25±0.59 | 4.28±0.47 | 4.13±0.34 | 0.023 | |
| SGOT (IU/L) | 23.30±6.82 | 24.28±6.40 | 28.15±35.75 | 22.38±9.55 | 20.23±7.54 | 0.010b, c | b-0.012, c-0.02 |
| SGPT (IU/L) | 22.65±8.73 | 21.98±5.82 | 20.97±8.78 | 21.95±11.25 | 18.50±6.93 | 0.007b, c | b-0.04, c-0.007 |
| T. Protein (gm/dl) | 7.37±0.90 | 7.20±0.92 | 7.43±0.93 | 7.34±0.76 | 7.37±0.74 | 0.814 | |
| Albumin (gm/dl) | 4.08±0.42 | 3.98±0.47 | 4.15±0.53 | 4.17±0.34 | 4.10±0.38 | 0.746 | |
| ALP (IU/L) | 96.60±34.39 | 92.55±32.43 | 98.60±34.94 | 99.68±36.10 | 94.25±39.34 | 0.078 | |
| iPTH (pg/ml) | 269.50±160.91 | 240.78±141.53 | 283.87±197.94 | 0.161 | |||
| FGF-23 (pg/ml) | 18.15±27.12 | 15.13±24.88 | 21.15±35.61 | 0.299* | |||
| Nutritional Intake: | |||||||
| T calories (kcal/day) | 1487.55±304.53 | 1472.25±127.55 | 1463.75±126.49 | 1535.13±158.32 | 1560.12±152.21 | 0.045 | |
| Proteins (gm/day) | 39.80±14.67 | 40.67±10.65 | 41.28±11.64 | 42.44±9.09 | 43.93±10.77 | 0.062 | |
| Phosphorous (mg/day) | 518.60±176.05 | 508.43±145.66 | 519.35±139.69 | 523.43±125.44 | 527.10±129.23 | 0.065 |
Data presented as Mean±SD. Hb- haemoglobin, TLC- total leucocyte count, eGFR- estimated glomerular filtration rate, iPTH: intact parathyroid hormone; ALP- alkaline phosphatase, FGF23- fibroblast growth factor 23, Computed with Friedman test
Group 3
Similar to groups 1 and 2, all the biochemical parameters did not change significantly, except serum phosphorus, calcium, FGF23, SGOT, and SGPT, which were significantly decreased over 12-months follow-up [Table 4]. The hemoglobin level also improved significantly at 12 months (P < 0.0001). The low dietary phosphorus intake was well balanced throughout the study period.
| Parameter | 0 month Mean±SD |
3 months Mean±SD |
6month Mean±SD |
9 months Mean±SD |
12 month Mean±SD |
P | P value (a, b, c) |
|---|---|---|---|---|---|---|---|
| HB (gm%) | 10.80±1.86 | 11.24±1.68 | 11.53±1.84 | 11.25±1.67 | 11.67±1.77 | <0.0001a, c | a-0.03, c-<0.0001 |
| Blood Urea (mg/dl) | 61.75±16.93 | 59.25±16.99 | 61.18±20.99 | 66.08±33.45 | 65.08±23.44 | 0.049 | |
| S.Creatinine (mg/dl) | 2.10±0.71 | 2.09±0.63 | 2.20±0.79 | 2.27±1.15 | 2.23±1.00 | 0.782 | |
| eGFR (ml/min) | 35.80±12.02 | 35.44±12.55 | 35.18±14.88 | 34.92±14.44 | 34.23±13.42 | 0.360 | |
| Calcium (mg/dl) | 8.85±0.70 | 8.88±0.67 | 8.80±0.76 | 8.82±0.60 | 8.55±0.60 | 0.018b, c | b-0.05, c-0.01, |
| Phosphorus (mg/dl) | 4.27±0.64 | 4.15±0.64 | 4.03±0.70 | 4.17±0.76 | 4.15±0.66 | 0.05c | c-0.07 |
| Sodium (mmol/l) | 138.35±4.92 | 138.77±3.30 | 139.50±2.91 | 139.05±3.61 | 140.35±3.03 | 0.004 | c-0.007 |
| Potassium (mmol/l) | 4.37±0.59 | 4.40±0.64 | 4.25±0.44 | 4.40±0.60 | 4.32±0.47 | 0.305 | |
| SGOT (IU/L) | 42.57±104.01 | 26.65±19.97 | 23.90±12.72 | 25.08±14.01 | 21.20±9.63 | <0.0001a, b, c | a-0.03, b-0.006, c-0.002 |
| SGPT (IU/L) | 41.37±97.18 | 25.35±15.78 | 20.55±8.62 | 21.45±15.75 | 17.55±6.29 | <0.0001a, b, c | a-0.001, b-0.01, c-<0.0001 |
| T. Protein (gm/dl) | 9.45±13.89 | 7.23±0.62 | 7.40±0.67 | 7.27±0.51 | 7.45±0.60 | 0.325 | |
| Albumin (gm/dl) | 3.88±0.56 | 3.93±0.46 | 4.10±0.50 | 4.06±0.34 | 4.00±0.23 | 0.099 | |
| ALP (IU/L) | 95.87±47.33 | 97.15±62.71 | 88.31±36.26 | 88.03±31.15 | 79.21±25.06 | 0.200 | |
| iPTH (pg/ml) | 288.15±167.15 | 273.00±155.03 | 275.90±153.29 | 0.622 | |||
| FGF 23(pg/ml) | 19.35±27.03 | 9.47±6.47 | 13.10±27.89 | 0.001*a, c | a-0.003, c-0.019 | ||
| Nutritional Intake*: | |||||||
| T calories (kcal/day) | 1489.33±294.80 | 1490.45±245.6 | 1470.00±139.27 | 1510.11±98.7 | 1525.13±129.06 | 0.204 | |
| Proteins (gm/day) | 40.77±14.90 | 41.23±15.44 | 43.35±19.62 | 40.94.88±10.46 | 42.05±9.86 | 0.071 | |
| Phosphorous (mg/day) | 517.28±178.99 | 519.44±190.78 | 520.15±235.38 | 505.24±177.65 | 504.67±118.21 | 0.81 |
Parametric Repetitive Measure ANOVA for the Computed with Friedman test; Data presented as Mean ± SD.Hb- haemoglobin, TLC- total leucocyte count, eGFR- estimated glomerular filtration rate, iPTH: intact parathyroid hormone; CRP: C reactive protein, ALP- alkaline phosphatase. Non Parametric Repetitive Measure ANOVA Computed with Friedman test for FGF23- fibroblast growth factor 23.
Relationship of FGF23 and phosphorus level
Based on FGF23, iPTH, and vascular calcification, patients in all the groups were further stratified into category 1, 2, and 3 according to the serum phosphorus levels of upto 3.9, 4–5, and >5mg/dl, respectively [Figure 3]. It was found that there was no significant relationship of FGF23 with different target values of serum phosphorus (P > 0.05).
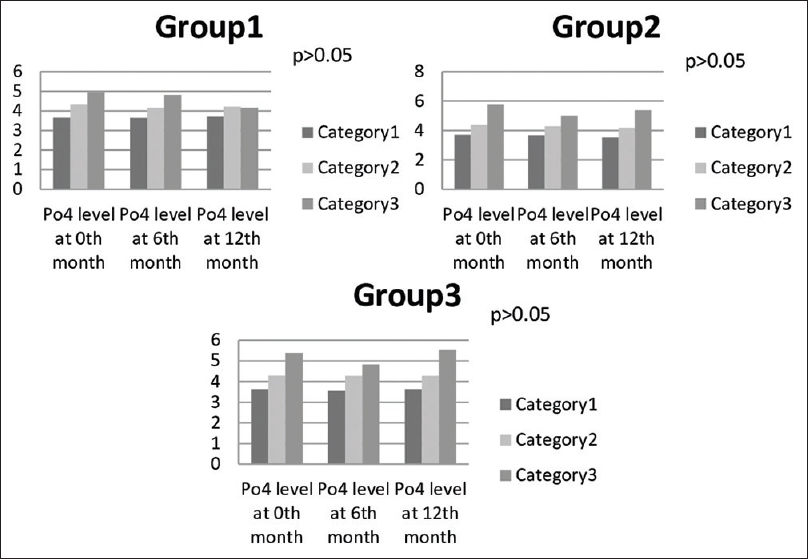
- Bar diagram showing relation of different phosphorus levels with progression of disease
Correlation of FGF23 with all parameters
The correlation of FGF23 with biochemical parameters, namely, calcium (P = 0.148), phosphorus (P = 0.132), ALP (P = 0.196), and albumin, was not significant. Similarly, the correlation with demographic parameters like gender, age, and mean blood pressure (BP) (P = 0.955) was not significant. However, eGFR of all patients and serum FGF23 were negatively correlated (r=−0.306, P = 0.001). Further, the correlation of FGF23 with iPTH and hemoglobin was significant (r = 0.199, P < 0.05).
Regression model for the progression of disease
The regression model was applied for the progression of disease, with eGFR as a dependent variable and FGF23, intact PTH, and phosphorus as independent variables. The results indicated that iPTH at baseline with an odds ratio (OR) of 0.215, (CI 0.050–0.922, P-0.039) and serum phosphorus at 12 months with OR 0.057 (CI 0.010–0.341, P-0.002) were the only independent variables that significantly influenced the progression of disease.
Discussion
In the present study, we assessed the target serum phosphorus level and its association with FGF23 in three different hyperphosphatemia management groups of stage 3 and 4 CKD patients. All three study groups, namely, phosphorus-restricted diet, calcium-based phosphorus binder, and non–calcium-based phosphorus binder, showed significant phosphorus reduction over 1 year follow-up. There was significant decline of FGF23 in non–calcium-based phosphate binder group, but not in the diet- and calcium-based phosphate binder groups. Serum phosphorus at the level of 5 mg/dl was associated with iPTH at baseline and with eGFR at 1 year; however, it was not associated with FGF23. Further, serum FGF23 levels were significantly correlated with iPTH and correlated negatively with eGFR. The latter may be pointing to a disrupted feedback loop resulting in high levels of serum FGF23.
The present study comprised middle-aged patients with slight male predominance. Age and gender did not show any correlation with FGF23 as per the literature, which also showed no age-related changes in intact FGF23 or in C-terminal FGF23.[10] Here, eGFR was on higher side (34.38 ± 11.84ml/min) compared to the Chronic renal Insufficiency Cohort (CRIC) study population's eGFR (32 ± 15 ml/min/1.73 m2).[11] Whether FGF23 contributes to CKD progression or is only a marker of its risk remains an important and unresolved question. However, some authors believe that FGF23 can be a novel predictor for the progression of CKD.[12] Similar to the previous study, the diabetic population formed 35.8% of the patients in our study.[11] It has been suggested by Titan et al.[13] that serum FGF23 is related to the risk of CKD progression in macroalbuminuric diabetic nephropathy (DN). But it is still unclear whether this relationship is causal. The baseline mean serum phosphorus levels were similar to a previous study in which the serum phosphorus was 4.2 ± 0.4 mg/dl.[14] Researchers are of the opinion that phosphate retention, and not FGF23, is the pathologic mechanism majorly involved in aging, glucose metabolism, insulin sensitivity disturbances, and oxidative stress.[15]
It was observed that there was a statistically significant reduction in serum phosphorus levels over 1 year in patients who were given non–calcium-based phosphate binders. In a previous study, it has been shown that sevelamer can diminish serum phosphate in dialysis patients.[16] In the study presented here, sevelamer treatment appeared to be effective in lowering serum FGF23 levels. It is possible that sevelamer's increased ability to reduce FGF23 occurs through an unknown or pleiotropic effect that is unrelated to the reduction of serum phosphorus.
Robust data is lacking on the role of dietary phosphate restriction in pre-dialytic CKD population. The present study showed a significant decline in serum phosphate levels in patients who were given phosphate-restricted diet, thereby reflecting that serum phosphorus is under the control of dietary intake. Similar findings were also reported in a study by Tsai et al.,[17] where a very low phosphate diet significantly lowered serum phosphate.
There was significant reduction in serum phosphorus levels in patients who were given calcium-based phosphate binders. The ability of calcium salts to effectively bind intestinal phosphate and reduce serum phosphate concentration has been recognized for decades. Similarly, in a controlled trial of three commercially available phosphate binders, Block et al.[14] reported a slight reduction in serum phosphate levels (mean serum phosphorus declined from 4.2 to 3.9 mg/dl over 9 months). Interestingly, we found that there was significant decline is serum calcium levels over a period of 12 months in patients who were given non–calcium-based phosphate binders, but similar decline was not seen in the group given calcium-based phosphate binder, thereby reflecting the impact of non–calcium-based phosphate binders on the levels of serum calcium in CKD population.
Serum FGF23 is considered an early biomarker of disordered mineral and bone metabolism in patients with CKD, as observed by Fliser et al.[12] They showed that FGF23 level was increased before an increase in PTH and phosphate was observed. However, in our study, secondary hyperparathyroidism was more prevalent than FGF23 excess. There was insignificant initial decline in serum FGF23 in group 1 patients over a period of 6 months, but not thereafter. The main explanation for this is that dietary phosphate-to-protein ratio restriction was not large enough in the study to achieve a dose–response FGF23-lowering effect. Additionally, the compliance of the patients to dietary restrictions cannot be ascertained. Chang et al.[18] also did not find any effect of a diet with low phosphate additives (11 mg/day) on FGF23 levels in patients with CKD stage 2. Isakova et al.[19] stated that phosphate-restricted diet did not significantly reduce FGF23 levels in early CKD. There were no significant treatment effects on the iPTH levels. Indeed, it may be because of relative hypocalcemia, which has led to elevated PTH levels. Similarly, in a 12-month study in CKD patients of stages 3a, 3b, and 4, there were no treatment effects on the iFGF23 or iPTH levels.[20]
FGF23 increased insignificantly from baseline to 12 months in group 2, possibly due to the exogenous calcium load that may have contributed to its elevation. Our results are as per a study of dietary phosphate restriction and therapy with lanthanum carbonate, in which the levels of FGF23 were not reduced.[19] Similarly, comparisons of lanthanum carbonate and placebo have yielded negative results in normo-phosphatemic, non-dialyzed patients with CKD.[21]
We determined the association between clinical variables of CKD progression and serum FGF23 concentration in patients with CKD in our study. FGF23 levels showed significantly strong correlation with eGFR. Though the correlation of FGF23 with intact PTH was significant and positively correlated, the correlation of FGF23 with calcium, phosphorus, ALP, and mean BP was not found to be significant. These results were as per a previous study which showed that FGF23 negatively correlated with eGFR (P-0.000) and iPTH (P-0.009) at baseline.[3] Similar to a previous study, there was no correlation of age, phosphorus, and nutritional phosphorus intake with FGF23 at early CKD.[7]
In our study, the correlation between iFGF23 and vascular calcification was not significant, which may be due to the younger population in the study in contrast to an observational French study. The authors in that study showed that aortic and coronary artery calcification scores could predict all-cause and cardiovascular mortalities in CKD patients.[22] Yuvaraj et al.[23] showed that raised intact-FGF23 was associated with cardiovascular mortality.
As an additional finding, there was progressive decline in SGOT/SGPT levels in patients who were given phosphate binders along with dietary phosphorus restriction. In our own study, SGOT/SGPT levels were low in hemodialysis patients; pyridoxine deficiency, increased levels of hepatocyte growth factor, uremic toxins, and hemodilution are the possible postulations.[24] Pleotropic effects of phosphate binder may be one of its etiologies; however, it needs further study.
This is thefirst Indian randomized controlled study conducted among three hypophosphatemia management groups taking dietary intervention to assess the phosphorus level based on FGF23 level. All participants were closely observed during the washout period before the intervention, and all were contacted telephonically to prevent any dietary deviations. The study cohort was small; it was from a single hospital, which may not extrapolate to other geographic regions. We have assessed bone disease based on noninvasive FGF23 levels, but not used the gold standard bone-biopsies, to assess bone turnover.
Conclusion
Serum phosphorus level upto 5mg/dl had no effect on FGF23 at early CKD stages; however, it was positively corelated with iPTH. Serum FGF23 levels were significantly positively correlated with iPTH and negatively correlated with eGFR. Although different treatment groups showed significant phosphorus reduction, non-calcium phosphate binders had a major impact on FGF23 reduction. Further studies are required considering dietary intervention without any phosphate binder for hyperphosphatemia management and to assess appropriate phosphorus level at every stage of early CKD in a larger long-term cohort.
Human and animal rights (with IRB approval number)
All procedures performed and planned in studies involving human participation were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted (vide TP (MD/MS) (13/2018)/IEC/PGIMER/RMLH 3008 dated November 28,2018) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are thankful to Prof. Yadunananda Prasad Gupta, statistician, and Prof. Venkatesan Sekhar for their contribution toward doing all statistical analyses.
References
- Global, regional, and national burden of chronic kidney disease, 1990–2017: Asystematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-33.
- [Google Scholar]
- Identification of high-risk population and prevalence of kidney damage among asymptomatic central government employees in Delhi, India. Saudi J Kidney Dis Transpl. 2016;27:362.
- [Google Scholar]
- Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737-47.
- [Google Scholar]
- Phosphate Metabolism in CKD Stages 3–5: Dietary and pharmacological control. Int J Nephrol. 2011;2011:970245.
- [Google Scholar]
- Executive summary of the 2017 KDIGO Chronic KidneyDisease-Mineral and Bone Disorder (CKD-MBD) guideline update: What's changed and why it matters. Kidney Int. 2017;92:26-36.
- [Google Scholar]
- Fibroblast growth factor 23 and parathyroid hormone after treatment with active vitamin D and sevelamer carbonate in patients with chronic kidney disease stage 3b, a randomized crossover trial. BMC Nephrology. 2012;13:49.
- [Google Scholar]
- A study on the association of serum fibroblast growth factor-23 with various indices of chronic kidney disease patients not yet on dialysis. J Renal InjPrev. 2016;5:104-7.
- [Google Scholar]
- A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461-70.
- [Google Scholar]
- 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA. 2014;311:507.
- [Google Scholar]
- FGF23 Is Not associated with age-related changes in phosphate, but enhances renal calcium reabsorption in girls. J ClinEndocrinolMetab. 2017;102:1151-60.
- [Google Scholar]
- Serum phosphate and mortality in patients with chronic kidney disease. CJASN. 2010;5:2251-7.
- [Google Scholar]
- Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The mild to moderate kidney disease (MMKD) study. JASN. 2007;18:2600-8.
- [Google Scholar]
- FGF-23 as a predictor of renal outcome in diabetic nephropathy. CJASN. 2011;6:241-7.
- [Google Scholar]
- A potential link between phosphate and aging – lessons from Klotho-deficient mice. Mech Ageing Dev. 2010;131:270-5.
- [Google Scholar]
- Fibroblast growth factor 23 in hemodialysis patients: Effects of phosphate binder, calcitriol and calcium concentration in the dialysate. NEC. 2011;117:c74-82.
- [Google Scholar]
- Short-term effects of very-low-phosphate and low-phosphate diets on fibroblast growth factor 23 in hemodialysis patients. Clin J Am SocNephrol. 2019;14:1475-83.
- [Google Scholar]
- Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:584-91.
- [Google Scholar]
- Effects of dietary phosphate and calcium intake on fibroblast growth Factor-23. Clin J Am SocNephrol. 2011;6:383-9.
- [Google Scholar]
- Changes in fibroblast growth factor 23 levels in normophosphatemic patients with chronic kidney disease stage 3 treated with lanthanum carbonate: Results of the PREFECT study, a phase 2a, double blind, randomized, placebo-controlled trial. BMC Nephrol. 2014;15:71.
- [Google Scholar]
- Randomized clinical trial of sevelamer carbonate on serum klotho and fibroblast growth factor 23 in CKD. Clin J Am SocNephrol. 2017;12:1930-40.
- [Google Scholar]
- Correlation of fibroblast growth factor 23 in chronic kidney disease patients with biochemical parameters and outcomes. J Parathyr Dis. 2015;4:7-10.
- [Google Scholar]
- Effects of pyridoxal phosphate in analysis of aminotransferase activity in patients undergoing hemodialysiseffects of pyridoxal phosphate in analysis of aminotransferase activity in patients undergoing hemodialysis. IJBAR. 2014;05:266-9.
- [Google Scholar]







