Translate this page into:
Effectiveness of a Low Dose Prednisolone Regimen for Treatment of Relapses in Children with Steroid Sensitive Nephrotic Syndrome
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
There may be a role of reducing the total steroid doses for the treatment of relapses of nephrotic syndrome in children with milder and more stable disease. The primary objective of this study was to compare the effectiveness of a low-dose prednisolone regimen with standard therapy for the treatment of relapses in steroid-sensitive nephrotic syndrome (SSNS) at the end of treatment, the secondary objectives being time to remission and sustained remission after 3 months.
Methods:
This randomized controlled trial included a total of 40 children (20 in each group) with SSNS (presently infrequently relapsing course) and with a relapse. Both groups received prednisolone at a dose of 2 mg/kg/day until remission; subsequently, the patients in the study group received 1 mg/kg, and the control group participants received 1.5 mg/kg prednisolone on alternate days for 4 weeks. The patients were followed up till 3 months after stopping the therapy.
Results:
The median (IQR) age of children enrolled was 7.5 (range: 5–9.65) years, and the age at onset of nephrotic syndrome was 4 (range: 2.3–5.5) years. The median time to achieve remission was 9 days (comparable in low dose vs. standard therapy group; P = 0.14). All patients were in remission at the end of therapy; 85% of patients were in the low-dose group and 90% in the standard therapy group after 1 month (P = 0.32). At the end of 3 months, 60% continued to be in remission in the low-dose group and 65% with standard therapy (P = 0.37). Hazard ratios for relapse at the end of 1, 2, and 3 months were 1.05, 1.08, and 1.13, respectively. Patients who were infrequently relapsing (79%) from the onset of nephrotic syndrome had higher remission rates at the end of 3 months (80% in the low-dose group vs. 76.9% in the standard therapy group). Hazard ratios for relapse in these patients at the end of 1, 2, and 3 months were 1.01, 1.03, and 1.08, respectively.
Conclusions:
Lower doses of prednisolone can be used for the treatment of relapse of steroid sensitive nephrotic syndrome, with an infrequently relapsing course.
Keywords
Relapse
steroid-sensitive nephrotic syndrome
steroid treatment
Introduction
Nephrotic syndrome (NS) is a chronic disorder in childhood with an annual incidence of 2–7/100,000 in children under 15 years of age, and its prevalence is around 12–16/100,000.[12] There is epidemiological evidence of a higher incidence in the Asian population.[3] More than 90% of children respond to treatment with oral corticosteroids, and about 60% have a frequently relapsing (FRNS) or steroid-dependent nephrotic syndrome (SDNS) that requires prolonged periods of steroid therapy or treatment with other immunosuppressants such as alkylating agents, levamisole, mycophenolate mofetil, and rituximab.[1]
Most guidelines suggest that the initial episode of NS be treated with prednisolone for at least total of 12 weeks with daily and alternate-day prednisolone for 6 weeks each and treatment of relapse for a duration of up to 6 weeks including daily and alternate-day regimens.[45]
The adverse effects associated with corticosteroids such as morphological changes (cushingoid facies, increased weight, and neck hump), increased predisposition to infections, impaired wound healing, hyperglycemia, ophthalmic complications (cataract, glaucoma), hypertension, short stature, and behavioral problems emphasize the need to minimize their use in children with NS.[6] Significant adrenocortical suppression has been shown to occur with even low doses of prednisolone administered on an alternate day basis.[7]
While various studies have been done for identifying the optimal duration of steroid therapy for the initial episode of NS, adequately powered trials looking at the treatment of relapses are limited. Also, the recommendation for treatment of relapses, whether infrequently relapsing (IFR) or FRNS/SDNS disease, is similar.[45] A retrospective study in children (n = 55) with SSNS (most patients were IFR) presenting with a relapse showed that a low-dose prednisolone regimen was successful in achieving remission in 70% of relapses without adversely affecting the relapse rate.[8] A randomized controlled trial in children (n = 30) looked at different doses of prednisone for inducing remission in relapses and concluded that doses of 1–1.5 mg/kg/day were sufficient for the same.[9] Another published RCT looked at different duration of steroids for treatment of relapses of SSNS in patients with FRNS/SDNS and atfirst relapse with doses of 60 mg/m2 of prednisolone till remission for 5 days followed by 40 mg/m2 doses over either 4 or 8 weeks. The study showed similar relapse rates at 6 months and concluded that there was no advantage of prolonging the same doses over longer periods.[10] A recently published RCT looked at the different doses for induction of remission (2 and 1 mg/kg) in children with all types of SSNS and found similar relapse rates in both groups at follow-up.[11] The present prospective randomized controlled study was planned to see if lower absolute doses of prednisolone can be used for treating relapses in children with SSNS, especially those with an infrequently relapsing course and maintaining remission subsequently. Body weight-based doses of prednisolone (2 mg/kg/day) were used for induction of remission and 1.5 mg/kg and 1 mg/kg on alternate days for maintaining remission in two different groups instead of body surface area-based calculation due to ease of use, and the slight under-dosing due to weight-based calculation does not seem to affect the outcomes.[1213] Besides the dose of prednisolone was reduced only after achieving remission as persistent relapse, it poses a significant risk of infections, thrombosis, and other complications.
Methods
This single-center, open-labeled, randomized controlled trial was done in the department of pediatrics of a tertiary care teaching hospital between March 2018 and March 2019. The study was approved by the institutional ethical committee and registered in the National Trial Registry (CTRI/2017/11/010376). The reporting of the study is as per the CONSORT statement.
All children and adolescents who were known cases of SSNS (IFR/SDNS or FRNS in the past) and had an infrequently relapsing course for the last 1 year and presenting with a relapse were enrolled for the study after obtaining parental consent/assent.
Nephrotic syndrome was defined as the presence of edema, urine protein/creatinine ratio >2, or 3+ or more protein on urine dipstick sample with serum albumin ≤2.5 g/dL and serum cholesterol >200 mg/dL. Disease remission was defined as urine protein nil or trace by urine dipstick on spot sample for 3 consecutive days and relapse as urine protein 3+ or more on spot sample for 3 consecutive days, having been in remission previously. Infrequently relapsing course (IFR) was defined as one relapse within 6 months of the initial response to steroids, or one to three relapses in any 12-month period; frequently relapsing disease (FRNS) as two or more relapses within 6 months of the initial response, or four or more relapses in any 12-month period; and steroid dependent as occurrence of two consecutive relapses during alternate day prednisolone therapy or within 2 weeks of cessation of treatment.[4]
Children with serious infections (sepsis, meningitis, peritonitis) and those receiving stress doses or on low-dose alternate-day prednisolone were excluded at the outset.
Patients presenting with a relapse of NS and who fulfilled the inclusion criteria were assessed for enrollment. Fifty-four children were assessed during the study period; a total of 40 children met the inclusion criteria and were further divided into two groups of 20 each.
Randomization
Computer-generated random numbers using block randomizations (blocks of 4) were used for allocating the intervention by a person not directly involved in the study. The interventions with serial numbers were kept in opaque sealed envelopes. Once the patient fulfilled the criteria for enrollment, he was assigned the group based on the intervention written inside the envelope. Both groups received prednisolone at a dose of 2 mg/kg/day until urine protein was trace or nil for 3 consecutive days. Subsequently, patients in the intervention group (low dose) received 1 mg/kg alternate-day prednisolone as a single morning dose for 4 weeks, and patients in the control group (standard dose) received 1.5 mg/kg alternate-day prednisolone in a similar manner.
A study questionnaire was filled out by all subjects and included baseline information such as the age of onset and diagnosis of NS, disease type, therapy received in the past, and relapse rate in the last 1 year. Examination was done, which included general physical and systemic examination and features of steroid toxicity (cushingoid facies, hypertrichosis, short stature). Any behavioral problems developing during the period of study were noted. The baseline investigations (blood urea, serum creatinine, total protein, serum albumin, globulin, and serum cholesterol) were done for all subjects. At the end of therapy, the proportion of children maintaining remission with both therapies was compared. Daily estimation of urine protein by urine dipstick test was done for all subjects till remission was achieved followed by twice a week urine protein estimation for the next 4 weeks (done by parents). Each patient's caregiver/parent was taught to do urine protein tests and was given a diary to maintain the daily record of urine protein and the prednisolone tablets administered. They were also advised to record any symptoms pertaining to infections or complications that would develop during the course of therapy. The subjects were monitored for remission 2 weeks after initiating the therapy and subsequently after 4 weeks. The patients were further followed up monthly till 3 months after stopping the therapy [Figure 1].
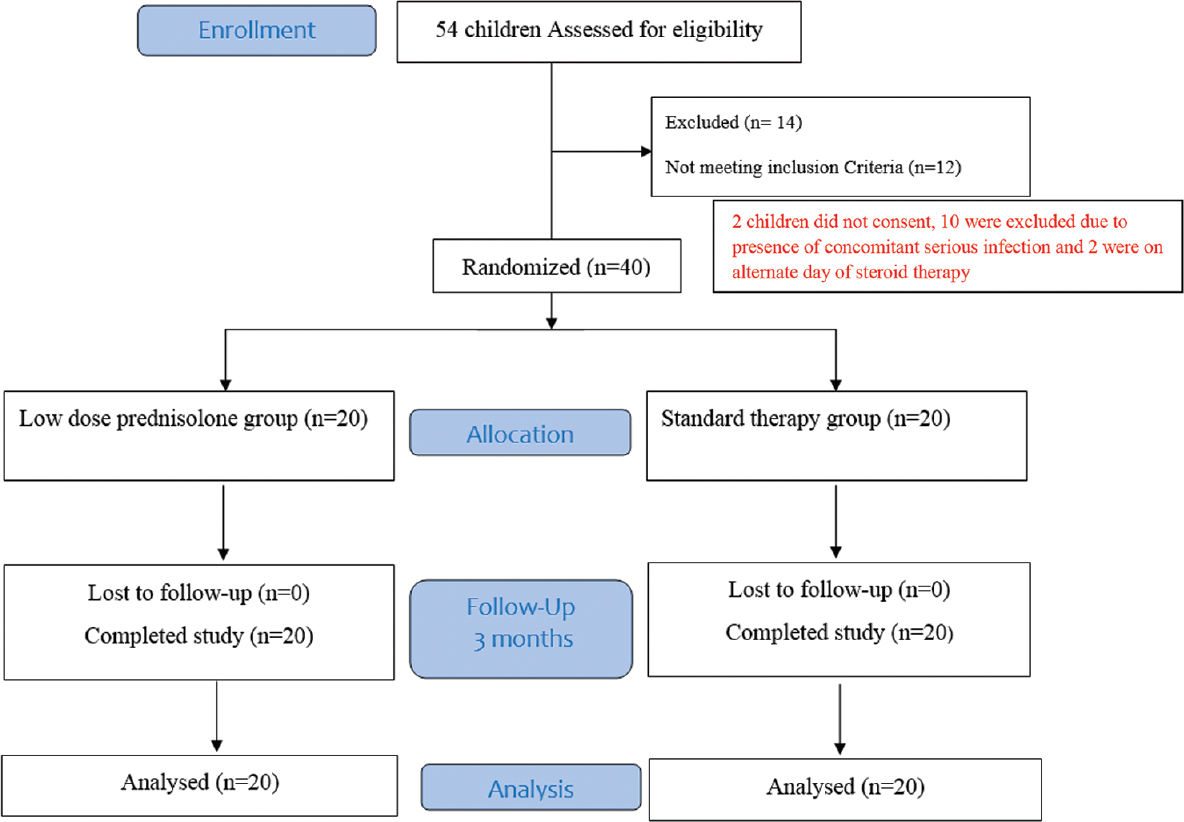
- The trial design
In addition, it was predefined that if the children develop any serious infection during treatment or experienced any severe complications of NS, they would be excluded from the study and treated with antibiotics, stress does of steroids, and other medications as required (depending upon the type of infection).
Outcomes
The primary outcome variable was the proportion of patients with remission in each group at the end of therapy, and the secondary variables were mean time to remission in each group, steroid doses received in the past 6 months, and relapses at 1 month and 3 months of follow-up. The side effects of the therapy were also compared. Remission was definite as urine protein nil or trace by dipstick for 3 consecutive days, while relapse was defined as the presence of urine protein of 2+ or more by dipstick with the presence or absence of edema.
Sample size and statistical analysis
The sample size was calculated for a non-inferiority trial with a significance level of 5%, power of study (1-beta) of 80%, percentage success defined as achieving remission at the end of prednisolone therapy in both control and experimental group of 95%, and equivalence limit d of 15%. The sample size thus calculated was 54, with 27 children in each group. However, due to the study being a single-center study and the inclusion criteria of infrequently relapsing course presently (for a uniform population), 40 children (20 in each group) could be enrolled during the study period. With this sample size, the equivalence limit reached 18% with all other parameters being the same.
Data entry was done using a Microsoft Excel spreadsheet and analyzed using descriptive statistics/SPSS version 22. Mean and standard deviation (SD)/median (IQR) were calculated for baseline characteristics such as age, age of onset, and biochemical parameters. Student t test or Mann–Whitney U test was applied for comparisons depending upon the use of mean or median for quantitative variables. Chi-square or Fisher exact test was used for categorical variables. For all comparisons, 5% probability (P < 0.05) was considered significant. Using Kaplan–Meier curve analysis, hazard ratio (HR) was calculated for thefirst relapse for both the groups and separately for infrequently relapsing patients separately.
Results
Baseline features
Of the 40 children enrolled, 34 (85%) were males; the median (IQR) age of the children enrolled in the study was 7.5 (range: 5–9.65) years, and the age at onset of NS was 4 (range: 2.3–5.5) years. Out of the total of 40 patients, 28 were IFR, 10 were FRNS, and two were SDNS in the past. The present course of all enrolled children was infrequent relapsers for the last 1 year. Of the 40 patients enrolled, 13 (32.5%) patients had relapses associated with infections. Eleven children had upper respiratory tract infections, one patient had urinary tract infection, and one had scalp pyoderma. Based on clinical features (periorbital puffiness, ascites, pedal edema), serum albumin, and use of diuretics, edema was categorized as mild, moderate, and severe. Twenty-three (57.5%) had mild edema, and 17 (42.5%) had moderate edema. The median (IQR) value of serum albumin was 2.4 (1.8, 2.6) g/dL. Twenty (50%) had serum albumin as 2.0–2.5 g/dL, and five (12.5%) had serum albumin <2.0 g/dL. None of the patients needed treatment for edema with the use of diuretics or albumin. Patients were given standard treatment for infections and were enrolled in the study after the treatment of infection if the relapse was still persistent.
The comparison of baseline characteristics, anthropometry, and biochemical parameters between the low-dose prednisolone and standard therapy groups are given in Table 1. The characteristics were similar in both groups. Most patients (70%) had received prednisolone alone, while the remaining 30% had received alternative agents in the past (not in the last 12 months). Twelve (25%) had received alkylating agents along with steroids; one (2.5%) patient each had received levamisole and cyclosporine.
| Parameter | Low dose steroid group n=20 Median (IQR) | Standard therapy group n=20 Median (IQR) | P |
|---|---|---|---|
| Males: Females | 18:2 | 16:4 | 0.37 |
| Age at presentation (years) Median (IQR) | 7 (2, 10) | 8 (4.8, 9.1) | 0.42 |
| Age at onset of disease (years) Median (IQR) | 4 (1, 5) | 4 (2.8, 5.3) | 0.84 |
| Type of SSNS in past (prior to last 1 year) | |||
| IFRNS | 15 (75%) | 13 (65%) | 0.5 |
| FRNS | 4 (20%) | 6 (30%) | 0.52 |
| SDNS | 1 (5%) | 1 (5%) | 1.0 |
| Weight SDS | −0.2 (−2.28, 0.658) | −0.18 (−0.55, 0.263) | 0.59 |
| Height SDS | −0.715 (−3.63, −0.715) | −1.3 (−1.88, −0.345) | 0.33 |
| BMI SDS | 0.515 (−1.46, 1.213) | 0.415 (−0.44, 1.593) | 0.46 |
| Blood pressure systolic (mm Hg) | 102 (90, 108) | 101 (88, 106) | 0.14 |
| Blood pressure diastolic (mm Hg) | 68 (64, 72) | 66 (63, 74) | 0.13 |
| Blood Urea (mg/dL) | 25 (14, 31) | 26 (24, 31) | 0.82 |
| Serum Creatinine (mg/dL) | 0.5 (0.3, 0.5) | 0.5 (0.4, 0.5) | 0.87 |
| Total Protein (g/dL) | 4 (4.2, 5.4) | 4.9 (4.6, 5.2) | 0.23 |
| Serum Albumin (g/dL) | 2.4 (1.8, 2.6) | 2.5 (2.2, 2.8) | 0.46 |
| Serum cholesterol (mg/dl) | 305 (258, 343) | 400 (352, 434) | 0.001* |
| Patients with infection associated relapses (%) | 6 (30%) | 7 (35%) | 0.37 |
| Relapses in last 1 year | 1 (0, 2) | 1 (0, 1) | 0.18 |
| Steroid dose in last 6 months (mg/kg/day) | 0.38 (0.28, 0.53) | 0.53 (0.38, 0.72) | 0.37 |
| Time to achieve remission | 9 (8, 10) | 9 (9, 10) | 0.14 |
Outcomes
All the patients were in remission at the end of therapy. The key outcomes are given in Table 2. The median time to achieve remission was 9 (8,10) days in the low-dose prednisolone group and 9 (9,10) days in the standard therapy group; P = 0.14. The mean prednisolone dose received in the low-dose group was 0.84 (0.05) mg/kg/day as compared to 1.31 (0.04) mg/kg/day in the standard therapy group (P = 0.02) over the treatment period lasting a maximum of 6 weeks.
| Key outcomes | Low dose steroid group n=20 | Standard therapy group n=20 | P |
|---|---|---|---|
| Proportion in remission at end of therapy | 20 (100%) | 20 (100%) | 1.0 |
| Proportion relapsing at 3 months | 8 (40%) | 7 (35%) | 0.37 |
| Mean Cumulative prednisolone doses for the current relapse | 0.84 (0.05) | 1.31 (0.04) | 0.02 |
Follow up (over next 3 months)
The patients were followed up monthly for 3 months after completing treatment for relapse. The monthly remission rates are provided in Table 3. At the end of 3 months, in the low-dose prednisolone group, 12 patients (60%) continued to be in remission, and in the standard therapy group, 13 (65%) patients were in remission. Of the 15 patients who relapsed in a 3-month period, seven (46.7%) had infection-associated relapses (six had acute respiratory tract infection and one had urinary tract infection). These acute respiratory tract infection-related relapses were all observed during the winter months and could be a seasonal factor for the relapses. The Kaplan–Meier curve HR for relapse at the end of 1, 2, and 3 months was 1.05 (0.35–3.1), 1.08 (0.36–3.14), and 1.13 (0.4–3.2), respectively [Figure 2].

- Kaplan−Meier curve for relapse-free survival till 3 months
| Low dose prednisolone group (N=20) | Standard therapy group (N=20) | P | |
|---|---|---|---|
| At end of therapy | 20 (100%) | 20 (100%) | 1.0 |
| At 1 month | 17 (85%) | 18 (90%) | 0.32 |
| At 2 months | 15 (75%) | 15 (75%) | 1.0 |
| At 3 months | 12 (60%) | 13 (65%) | 0.37 |
While all the 40 patients at the time of enrolment had an IFRNS course, in the past, some of them (30%) had behaved as FRNS or SDNS. The baseline characteristics and outcomes of patients in both groups who were infrequently relapsers (70%) to begin with were further compared. The baseline characteristics were similar in both groups except for serum cholesterol, which was higher in patients treated in the low-dose steroid group (P = 0.007). A higher proportion of patients in both groups were in remission at the end of 3 months after completion of therapy (93.3% vs. 92.3%). The Kaplan–Meier curve HR for relapse was calculated for IFR patients in both groups. At the end of 1, 2, and 3 months, HR was 1.01 (0.3–2.1), 1.03 (0.32–2.3), and 1.08 (0.35–2.3), respectively [Figure 3].

- Kaplan−Meier curve for relapse-free survival till 3 months in children with IFR course from the disease onset in both groups
A comparison of the baseline characteristics of patients who maintained remission versus the patients who relapsed at the end of 3 months showed that patients who had onset of disease at a younger age (4.4 vs. 6.4 years; P = 0.03) and higher relapse rate (1.28 vs. 0.72; P = 0.013) in the last 1 year were more likely to relapse. Also, the proportion of infection-associated relapses was higher in patients who had relapsed at the end of 3 months (53.3% vs. 20%; P = 0.014).
Adverse effects of steroids
Thirteen (32.5%) patients showed adverse effects; of these, 10 (25%) complained of hyperphagia, eight (20%) had symptoms of myalgia, seven (17.5%) had cushingoid facies, and four (10%) developed hypertension. The distribution of these events was comparable in both groups for all these symptoms and signs [Table 4].
| Parameter | Low dose steroid group (N=20) | Standard therapy group (N=20) | P |
|---|---|---|---|
| Hyperphagia | 5 (25%) | 5 (25%) | 0.5 |
| Myalgia | 3 (15%) | 5 (25%) | 0.22 |
| Cushingoid facies | 2 (10%) | 5 (25%) | 0.11 |
| Hypertension | 1 (5%) | 3 (15%) | 0.15 |
Discussion
In the present study, all enrolled patients had SSNS with an infrequently relapsing course for the past 1 year. Most (70%) had infrequent relapses since the beginning of the disease, while the remaining had FRNS/SDNS course in the past. The median (IQR) relapse rate of the study population for the last 1 year was 1 (0,2) relapse. In a study done by Karnika et al.[6] where lower doses of prednisolone (1 mg/kg/day) were used for inducing remission in SSNS children during relapses, the mean number of relapses in the previous 6 months was 1.0 ± 1.1. As all enrolled patients in our study had an IFR course in the recent past, the mean steroid doses received in the last 6 months were also minimal (0.04 mg/kg/day).
The baseline features, anthropometry, and biochemical parameters of the patients in the two groups were found to be comparable; except for serum cholesterol (P = 0.001), no statistically significant difference was found in both groups. Similar rates of infection-associated relapses were also observed in both groups (30% in the low-dose group and 35% in the standard therapy group; P = 0.37). The median time to achieve remission for all patients was 9 days; it was similar in both groups (P = 0.14). Another RCT in children (n = 30) that looked at varying doses of prednisolone for inducing remission in SSNS relapses (2, 1.5, and 1 mg/kg/day) showed mean time to remission as 7.2, 10.2, and 9 days, respectively, in these groups.[7] Similar times to remissions were also reported by another RCT.[11] However, in the present study, same doses (2 mg/kg/day) of prednisolone were used for inducing remission and the steroid doses were reduced thereafter.
All patients were in remission at the end of therapy. At 1 month after completion of treatment, 85% of patients were still in remission in the low-dose prednisolone group as compared to 90% in the standard therapy group (P = 0.32). At the end of 3 months, in the low-dose prednisolone group, 60% continued to be in remission, and in the standard therapy group, 65% of patients were in remission (P = 0.37). At the end of 1, 2, and 3 months, the HRs for relapses were 1.05, 1.08, and 1.13, respectively.
Though the proportions of patients with sustained remission at 3 months follow-up were comparable in both the groups (60% vs. 65%), the proportion of patients in the study population who relapsed at the end of 3 months overall was found to be higher (37.5%). The reason for the high incidence of relapse could be infection-triggered relapses during that season; of the 15 patients who relapsed in a 3-month period, seven (46.7%) had infection-associated relapses. Most of these patients had an upper respiratory tract infection. Relapses associated with or triggered by infections are more common in developing countries as compared to the western world.[1415]
We also compared the baseline characteristics and outcomes in patients who were infrequent relapsers since the onset of NS in the low-dose and standard therapy groups. It was observed that the proportions of patients with sustained remission were similar at the end of 1 and 3 months after the completion of therapy (93.3% vs. 92.3% and 80% vs. 76.9%, respectively). At the end of 1, 2, and 3 months, Kaplan–Meier curve HR for relapse was 1.01, 1.03, and 1.08, respectively, indicating similar response rates in both the groups. These better remission rates in IFR were probably due to a milder course of the disease.
The mean prednisolone dose received for the treatment of current relapse was 0.84 mg/kg/day in the low-dose group as compared to 1.31 mg/kg/day in the standard therapy group (P = 0.02), indicating a significant dose reduction using this regimen. Similar observations have been made in the previous RCT too, though the sample size in each group in that study was rather small.[7] For total dose calculation, steroid doses for the entire treatment period were used (including the daily and alternate-day doses).
The adverse effects of steroids were comparable in both groups; the proportion of patients who complained of hyperphagia was similar (P = 0.5). Though the proportions of patients who developed cushingoid facies, myalgias, and hypertension were higher in the standard therapy group, the difference was not statistically significant. The median SDS scores of weight, height, and BMI of the children were −0.18, −0.97, and 0.47, respectively, indicating that most patients were growing normally. Five (12.5%) children had height SDS below − 2, which is comparable to a study by Ishikura et al.[16] where short stature as an adverse effect attributed to steroids was seen in 13% of patients.
Further, it was observed that patients who had a younger mean age at onset of disease (4.4 years) were more likely to relapse with a low-dose prednisolone regimen as compared to patients who had onset of disease later in life (6.42 years). Age younger than 3 years at onset of NS is more likely to predispose to FRNS/SDNS course of the disease.[17] Also, the number of infections was higher in the patients who relapsed (53.3% vs. 20%), and most (84.6%) were upper respiratory tract infections.
A major limitation of the present study was the small sample size and a shorter (3 months) follow-up period post therapy, which is often insufficient to define the course of NS, especially the infrequent relapsers. However, the baseline relapse rate of the enrolled patients in the previous year was low, and it may be assumed that they were likely to behave in a similar manner in the subsequent year too. As we wanted to look at the effect of lower doses in milder patientsfirst, the inclusion criteria became stricter; patients only with an IFR course in the past 1 year were thus enrolled. Being a single-center study, it was difficult to enroll a larger number of patients over a short period of time with such criteria. Hopefully, multicentric trials with larger cohorts and longer duration of follow-up in the future would be able to better define the appropriate doses and duration of treatment for relapses in different categories of patients (IFR or SDNS/FRNS).
Conclusions
Based on the results of our study, we conclude that low-dose prednisolone regimen was comparable to the current standard therapy in the treatment of relapses of SSNS. Though it resulted in similar proportions of remission and relapse rates at the end of 3 months, the remission rates were better for patients who were IFR since disease onset. While the adverse events were comparable statistically, the proportion of patients who became cushingoid, experienced myalgias, and developed hypertension were more in the standard therapy group.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nephrotic syndrome in The Netherlands: A population-based cohort study and a review of the literature. Pediatr Nephrol. 2011;26:1241-6.
- [Google Scholar]
- High incidence of minimal change nephrotic syndrome in Asians. Arch Dis Child. 1985;60:1018-20.
- [Google Scholar]
- Steroid sensitive nephrotic syndrome: Revised guidelines. Indian Pediatr. 2021;58:461-81.
- [Google Scholar]
- Management of complications of glucocorticoid therapy. Clin Chest Med. 1997;18:507-20.
- [Google Scholar]
- Adrenocortical suppression in children with nephrotic syndrome treated with low-dose alternate day corticosteroids. Indian J Nephrol. 2018;28:203-8.
- [Google Scholar]
- Use of a low-dose prednisolone regimen to treat a relapse of steroid-sensitive nephrotic syndrome in children. Pediatr Nephrol. 2017;32:99-105.
- [Google Scholar]
- Lower prednisone dosing for steroid-sensitive nephrotic syndrome relapse: A prospective randomized pilot study. Eur J Pediatr. 2020;179:279-83.
- [Google Scholar]
- Results of the PROPINE randomized controlled study suggest tapering of prednisone treatment for relapses of steroid sensitive nephrotic syndrome is not necessary in children. Kidney Int. 2021;99:475-83.
- [Google Scholar]
- Low-dose versus conventional-dose prednisolone for nephrotic syndrome relapses: A randomized controlled non-inferiority trial. Pediatr Nephrol. 2021;36:3143-50.
- [Google Scholar]
- Body weight-based prednisolone versus body surface area-based prednisolone regimen for induction of remission in children with nephrotic syndrome: A randomized, open-label, equivalence clinical trial. Pediatr Nephrol. 2016;31:595-604.
- [Google Scholar]
- Efficacy of body weight vs body surface area-based prednisolone regimen in nephrotic syndrome. Clin Exp Nephrol. 2020;24:622-9.
- [Google Scholar]
- Infection associated relapses in children with nephrotic syndrome: A short-term outcome study. Saudi J Kidney Dis Transplant. 2019;30:1245-53.
- [Google Scholar]
- Infections in children with nephrotic syndrome. J Coll Physicians Surg Pak. 2003;13:337-9.
- [Google Scholar]
- Morbidity in children with frequently relapsing nephrosis: 10-year follow-up of a randomized controlled trial. Pediatr Nephrol. 2015;30:459-68.
- [Google Scholar]
- Influence of age at onset on the outcome of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 1998;12:467-70.
- [Google Scholar]







